Introduction
Solitary Fibrous Tumor (SFT) is an uncommon benign neoplasm that arises from mesenchymal tissue. Initially documented by Klemperer and Rabin in 1931 [1], it was believed affect
only pleura and peritoneum, it is now recognized in various locations, including the oral cavity [2]. Oral SFT can develop in
individuals of all ages and typically affects female adults [2].
Based on the most recent categorization by the World Health
Organization (WHO) for head and neck tumors, SFT is categorized as a borderline/low-grade mesenchymal tumor [3]. From a
clinical standpoint, it is not possible to distinguish it from other
reactive and neoplastic lesions of the oral cavity. As it is referred
“the many face tumor” in some papers [4], it shows very similar
histological features with other soft tissue tumors. Thus, the diagnosis should rely on clinical, histomorphological, Immunohistochemical (IHC), and molecular findings. The objective of this
research is to report an uncommon occurrence of a SFT in the
oral cavity and to highlight the significance of IHC and molecular
analysis in differential diagnosis.
Case report
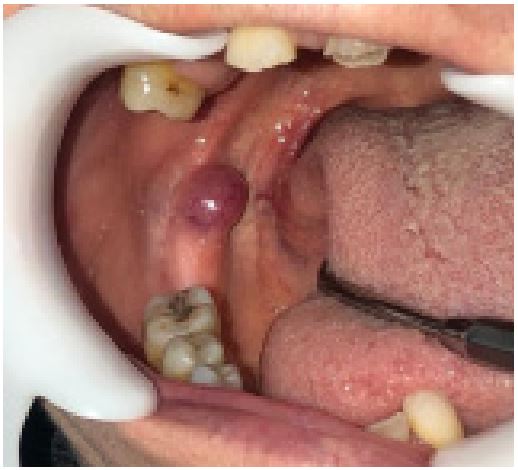
A 63-year-old female patient presented to the Oral and Maxillofacial Radiology Department needing dentures. The patient
had a medical background of systemic hypertension and diabetes mellitus and adhered to her medication schedule consistently. The intraoral examination revealed a pedunculated, vascularized, painless nodular exophytic mass with a rubbery-firm
texture. The mass measured 1.4 cm in its largest dimension and
was located in the retromolar region of the mandible (Figure
1). The patient stated that she had no awareness of the lesion
and had not experienced any previous traumas. The extraoral
examination yielded normal results. The preliminary diagnosis
was a traumatic fibroma. The lesion was excised under local anesthesia, fixed in 10% buffered formalin, and sent to the Department of Oral Pathology.
Grossly, the specimen was 1.4 x 0.9 x 0.7 cm in size with an
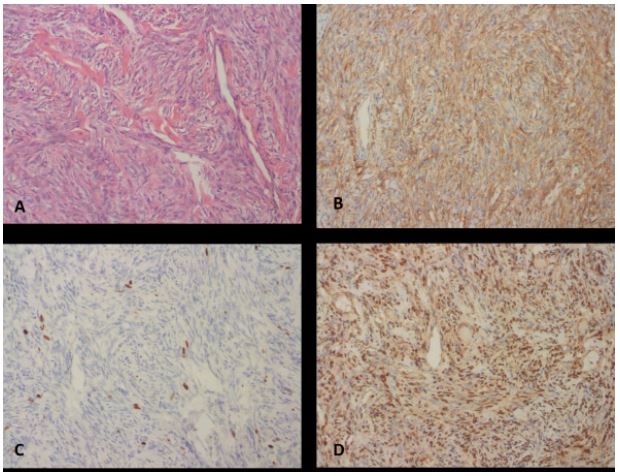
encapsulated, whitish, firm-cut surface. Histopathological examination revealed the proliferation of spindle cells with thin fibrous encapsulation. The tumor cells had fusiform nuclei with
a pale cytoplasm and no atypia. There was no evidence of mitosis or necrosis. The spindle-shaped tumor cells in a storiform
pattern and thin-walled, branching-staghorn pattern vascular
structures were observed in the collagenous stroma (Figures
2A,2B). To distinguish from soft tissue sarcomas, an IHC panel
and FISH were conducted. The tumor exhibited positive immunoreactivity for CD34 and STAT6, while negative for S-100 and
beta-catenin. ki67 proliferation index was less than 1%. Furthermore, using chromosome 18q11.2 as a translocation partner,
sections from paraffin blocks were examined for chromosomal
translocations by FISH with dual-color, break-apart probes. SYTtranslocation was yielded negative. Based on these results, the
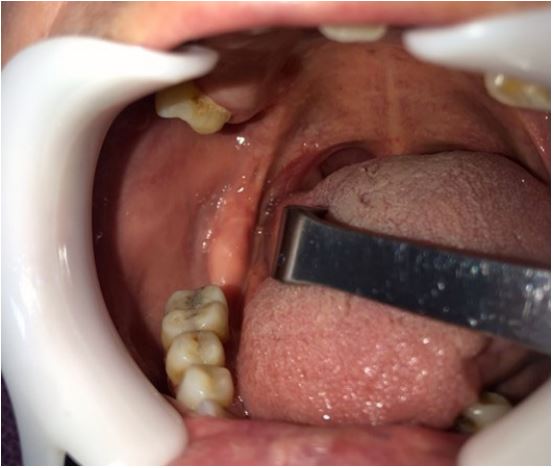
final diagnosis was oral SFT. There was no recurrence detected
three months after removal (Figure 3).
Discussion
SFT is a unique tumor originating from fibroblasts, initially
found in the pleural cavity, with potential to develop in any anatomical site [1]. Extrapleural cases, affecting 27% of the head
and neck region, can be observed in various locations such as
the orbit, nasal cavity, paranasal sinuses, thyroid, and salivary
glands [5]. SFTs in the oral region are extremely uncommon and
often impact the buccal mucosa, tongue and hard palate [6].
SFT rarely occurs in the retromolar region. Recent research estimates a 2.6% incidence of SFT in the retromolar area, with a total of 4 reported cases [7]. To the author’s knowledge, this case represents the fifth case of SFT occurring in this area. Lesions
are usually characterized by well-defined submucosal growths
that might vary in size and duration. The color and texture of
the covering mucosa are often uniform [2,6]. Pain in oral SFTs
is rarely documented, and ulceration is often caused by local
trauma [7]. In this case, there was an elevation in mucosal appearance without local trauma, resembling those described in
existing literature.
SFTs are histologically unique neoplasms, and the heterogeneity of their microscopic features can be somewhat challenging to diagnose. The diversity of morphological features and
the “patternless” growth pattern pose diagnostic challenges for
SFT and require differential diagnosis with benign and malign
mesenchymal tumors. Oral SFT is histologically characterized
by a varying density of spindle tumor cells, extensive collagen
deposition, and thin-walled blood vessels resembling hemangiopericytoma. There is no evidence of cytological atypia or necrosis [8]. The histopathological characteristics of our case were
comparable to those described in the literature. It is essential to
differentiate oral SFTs from other soft tissue lesions, as they can
range from a simple traumatic fibroma to a synovial sarcoma
[4], due to their non-specific clinical presentation and diverse
histologic characteristics [5]. Oral SFTs are often diagnosed using IHC analysis employing CD34, CD99, and Bcl-2 markers [9]
or identifying the chromosomal rearrangement NAB2-STAT6
genes with Fluorescent In Situ Hybridization (FISH) [10,11].
SFT is predominantly characterized by a positive expression of
CD34, while there are occasional instances when it may exhibit
a negative expression. Hence, it is advisable to utilize markers
such as CD99 and Bcl-2 in conjunction with CD34. In a recent
research [2], it was observed that 72% of oral SFTs had CD99
positivity. However, this particular case was found to be CD99
negative. The S-100 protein tested negative, consistent with the
findings reported in the literature [2,8]. By utilizing molecular
tools, an intrachromosomal fusion involving NAB2 and STAT6
genes at the 12q.13 locus was identified by DNA sequencing
[5,12]. The consistent presence of the NAB2-STAT6 fusion gene
in nearly all instances of SFT suggests that this genetic alteration is the main cause of SFT development, regardless of the location and appearance of the tumor [5]. According to the literature, SFT demonstrates STAT6 positive in almost 99% of cases
[13,14]. Corroborating these results, this case exhibited strong
immunopositivity for STAT6. Given the histological similarities,
it was crucial to differentiate from synovial sarcoma, which can occur in the oral region. FISH is widely regarded as the most
dependable technique for detecting synovial sarcoma due to its
ability to detect SYT-SSX translocation fusion genes at the molecular level. According to the literature, FISH has been found
to yield accurate results in 82% of synovial sarcoma [15]. In our
case, the results of beta-catenin immunostaining and the presence of SYT-SSX fusion genes were both negative, leading us to
dismiss synovial sarcoma from the list of possible diagnoses.
The literature indicates that excision is sufficient in the case
of oral lesions [2,4]. The existence of malignancy in SFTs is typically indicated by the patient’s advanced age, a large tumor
size, and malignant histological characteristics [4,5]. While malignant oral SFT is rare, it is nonetheless possible. Regarding our
case, the tumor size was small, with mitotic activity measuring
less than 1%. Despite the low likelihood of malignancy in our
case, the patient is being followed-up for 3 months, with no recurrence or evidence of malignant transformation.
Conclusion
To conclude, we reported an uncommon case of SFT in the
retromolar region. We discussed the clinical, histopathological,
and molecular features. The correct diagnosis is crucial for the
appropriate treatment and management of SFTs. Given its rarity
in the oral cavity, differential diagnosis with other lesions in the
oral mucosa and periodic follow-up are considered necessary.
Declarations
Conflicts of interest: None.
Authors contributions: SKY and MT examined the patient
and performed treatment and follow-up. SEG and IAS carried
out the histological analysis. The laboratory procedures were
executed by IAS. First draft of the paper was written by IAS and
SKY. SEG and MT reviewed the manuscript’s final version.
References
- Klemperer P. Primary neoplasms of the pleura: a report of five cases. Arch Pathol. 1931; 11: 385-412.
- Nunes FB, Sant’Ana MSP, Silva AMB, Agostini M, Silva Canedo NH, et al. Solitary fibrous tumour of the oral cavity: An update. J Oral Pathol Med. 2020; 49(1): 14-20.
- Sbaraglia M, Bellan E, Dei Tos AP. The 2020 WHO classification of soft tissue tumours: News and perspectives. Pathologica. 2021; 113(2): 70.
- Tariq MU, Din NU, Abdul-Ghafar J, Park YK. The many faces of solitary fibrous tumor; diversity of histological features, differential diagnosis and role of molecular studies and surrogate markers in avoiding misdiagnosis and predicting the behavior. Diagn Pathol. 2021; 16(1): 32.
- Ronchi A, Cozzolino I, Zito Marino F, Accardo M, Montella M, et al. Extrapleural solitary fibrous tumor: A distinct entity from pleural solitary fibrous tumor. An update on clinical, molecular and diagnostic features. Annals of Diagnostic Pathology. 2018; 34: 142-150.
- Alawi F, Stratton D, Freedman PD. Solitary fibrous tumor of the oral soft tissues: A clinicopathologic and immunohistochemical study of 16 cases. The American Journal of Surgical Pathology.2001; 25(7): 900-910.
- de Morais EF, Moreira DG, Oliveira VA, Rodrigues RR, Germano AR, et al. An Unusual Clinical Presentation of Solitary Fibrous Tumor in the Oral Cavity. Case Rep Pathol. 2017; 2017: 4395049.
- Lo Muzio L, Mascolo M, Capodiferro S, Favia G, Maiorano E. Solitary fibrous tumor of the oral cavity: the need for an extensive sampling for a correct diagnosis. J Oral Pathol Med. 2007; 36(9): 538-542.
- Carlos R, de Andrade BA, Canedo NH, Abrahao AC, Agostini M, et al. Clinicopathologic and immunohistochemical features of five new cases of solitary fibrous tumor of the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016; 121 (4): 390-395.
- Robinson DR, Wu YM, Kalyana-Sundaram S, Cao X, Lonigro RJ, et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet. 2013; 45(2): 180-185.
- Chmielecki J, Crago AM, Rosenberg M, O’Connor R, Walker SR, et al. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet. 2013; 45(2): 131-132.
- Cheah AL, Billings SD, Goldblum JR, Carver P, Tanas MZ, et al. STAT6 rabbit monoclonal antibody is a robust diagnostic tool for the distinction of solitary fibrous tumour from its mimics. Pathology. 2014; 46(5): 389-395.
- Tai HC, Chuang IC, Chen TC, Li CF, Huang SC, et al. NAB2-STAT6 fusion types account for clinicopathological variations in solitary fibrous tumors. Mod Pathol. 2015; 28(10): 1324-1335.
- Mohajeri A, Tayebwa J, Collin A, Nilsson J, Magnusson L, et al. Comprehensive genetic analysis identifies a pathognomonic NAB2/STAT6 fusion gene, nonrandom secondary genomic imbalances, and a characteristic gene expression profile in solitary fibrous tumor. Genes Chromosomes Cancer. 2013; 52(10): 873-886.
- Ten Heuvel SE, Hoekstra HJ, Suurmeijer AJ. Diagnostic accuracy of FISH and RT-PCR in 50 routinely processed synovial sarcomas. Applied Immunohistochemistry & Molecular Morphology. 2008; 16(3): 246-250.