Background
Neuromodulation, which involves modifying nerve activity
by directly applying electrical or pharmacological agents to specific regions, has been at the forefront of medical therapeutic
research and innovation for many years [1]. Within the spectrum of neuromodulatory instruments, Spinal Cord Stimulators
(SCS) distinguish themselves, showcasing significant strides in
therapeutic progress and proven efficacy in clinical settings [2-
10]. The concept of SCS emerged in the 1960s to address intractable pain. The publication of the Gate Control Theory in 1965,
Annals of Surgical
Case Reports & Images
which revealed pain as a complex interplay within the nervous
system, shifted the medical approach from irreversible nervedamaging surgeries to reversible treatments like neuromodulation [10].
Fundamentally, these devices are designed to modulate neural activities within the spinal cord [1]. Through the precise delivery of electrical impulses, they not only mitigate symptoms of
chronic pain but also possess the potential to modify neuroplastic changes associated with chronic pain states [3]. Strategically
positioned electrodes near the spinal cord intercept nociceptive signals, modulating their transmission and consequently attenuating the perception of pain at the cortical level [3].
While the primary indication for SCS has been chronic pain
syndromes, including post-laminectomy syndrome, complex regional pain syndrome, and neuropathic pain, the versatility of
this device has led to its exploration in various other conditions
[4]. From ischemic conditions like angina to functional disorders
like urinary incontinence and even certain types of migraines,
the expanding scope of SCS applications is a testament to its
therapeutic potential and adaptability [5]. The paradigm shift in
the understanding of chronic pain, coupled with technological
advancements, has placed the SCS at the forefront of neuromodulatory interventions. Further, modern SCS systems offer
advanced features such as wireless connectivity, customizable
pulse outputs, and rechargeable power sources, making SCS
systems more efficient, effective, and user-friendly [2].
Unlike the chronic pain conditions usually treated with SCS,
compartment syndrome poses a distinct clinical challenge due
to its nature of acute or chronic increase in pressure within
muscle compartments, leading to severe clinical difficulties [6].
This heightened pressure can impede blood flow, potentially
leading to tissue damage, necrosis, and nerve injury. Contributing factors include severe injuries, bone fractures, sustained
immobility, or vigorous physical activities [6]. While acute cases
often demand emergent interventions, chronic forms of the
syndrome, though less immediately life-threatening, present
a unique set of challenges in terms of management and longterm prognosis [7]. Traditionally, surgical fasciotomy has served
as the primary treatment for compartment syndrome, designed
to relieve elevated pressure within compartments and reinstate
blood flow. Yet, not every patient experiences enduring relief
from this invasive method. Considering the possible complications and the increased risk of morbidity from multiple surgeries, the need for alternative treatment approaches is clear [8].
Connecting the realms of SCS and compartment syndrome
is the pioneering promise of neuromodulation. The 31-yearold female patient underwent a percutaneous stimulation trial
using a sophisticated neuromodulatory approach for treating
compartment syndrome. The device utilized was the Abbott
Medical Penta 3 mm Lead, 60 cm; SCS Paddle Lead (Plano, TX,
USA), known for its small electrodes arranged in a five-column
array. The configuration enhances the ability to manage complex pain by providing advanced control over the affected areas. This innovative fusion of technology and clinical practice
suggests a more comprehensive approach to care, enhancing
traditional treatments with advanced techniques.
Case
A 31-year-old female with a primary medical history that
includes generalized anxiety, chronic depression, mild intermittent asthma, IBS, dyslipidemia, and bipolar disorder consulted
her Primary Care Physician (PCP) due to pain in her left lower
extremity. She maintained a healthy lifestyle as a non-smoker
and non-drinker but had a BMI of 41.
Her symptoms began when she increased her training intensity, running longer distances than she was used to. Initially
diagnosed with a sprain in her left foot, subsequent imaging
showed a stress fracture, which was managed conservatively.
Despite this, she continued her training and began experiencing
cramps in both calves while running, more pronounced on the
left. Though physical therapy and medications offered momentary relief, the problem persisted.
Throughout this period, her laboratory results and vitals
stayed largely within the normal range. Detailed imaging studies, including a doppler of her lower extremities and an evaluation of her popliteal arteries, showed no abnormalities. However, compartment pressure testing revealed elevated pressures
in her left anterior (72) and lateral (36) compartments following exercise, with resting values of 14 and 13 respectively. The
right side also showed slightly raised pressures. These findings
confirmed a diagnosis of chronic exertional compartment syndrome in both lower extremities. After discussing her options,
she chose to undergo a bilateral anterior and lateral fasciotomy
of the lower extremities, which brought about a significant reduction in her pain, with no major post-operative complications.
Approximately eighteen-months post-procedure, she began
experiencing a recurrence of pain in her left lower extremity,
reminiscent of her prior exercise-induced discomfort. Subsequent discussions with her orthopedic surgeon culminated in a
revision surgery for the left leg’s anterior and lateral compartments. The patients pain intensified post-surgery and persisted
for about a year, however, her right leg responded well to the
initial surgery and remained asymptomatic.
Persisting pain in her left leg was described as sharp, piercing, occasionally accompanied by numbness and weakness. An
MRI of her lumbar spine showed no anomalies, while an MRI
of her femur/tibia identified nonspecific muscle edema in her
left mid-leg, distinct from previous MRIs. Potential causes included exercise-induced trauma, delayed-onset muscle soreness, denervation edema, inflammatory myopathies, or possibly an infectious/viral myopathy. An EMG ruled out neuropathy
or myopathy. While there was some consideration for a CRPS
diagnosis due to observed allodynia and temperature changes,
she didn’t meet the complete Budapest criteria. She underwent
Botox injections under the guidance of a sports medicine physician to manage her chronic compartment syndrome, but their
efficacy diminished over time.
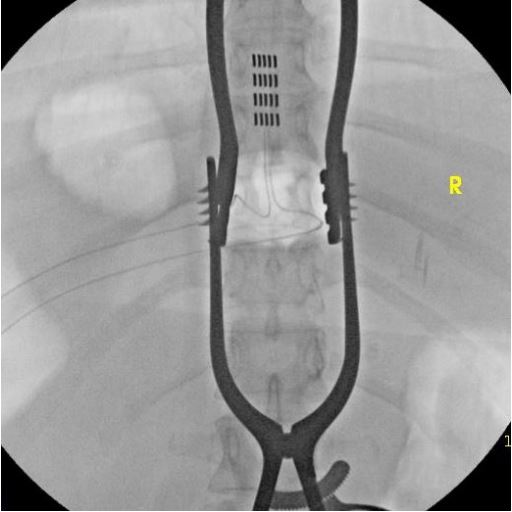
Five years post her initial injury, the patient underwent a SCS
trial which yielded promising results. She experienced a notable
70% decline in pain during the trial, which further improved to
over 90%. Given the significant relief, she elected to have a permanent spinal cord stimulator implanted (Figures 1a, 1b). At her
six-week surgery follow up, the patient continued to fare exceptionally well, still reporting a relief of >80% from her pre-surgical
pain levels. Approximately three-months following the revision
surgery, the patient reports a significant improvement in pain
and quality of life following the implantation of a permanent
spinal cord stimulator. Objective measures indicated that her
daily movement and step count, as monitored by a wearable
device, increased twofold, suggesting enhanced mobility. The
patient’s self-reported pain levels decreased from a baseline of
7-8 (on a 10-point visual analog scale) to not exceeding a level
of 3 post-surgery, denoting >90% reduction in pain perception.
Subjectively, the patient described a substantial uplift in
cognitive clarity and energy levels, contrasting her pre-implant
state characterized by fatigue and ‘brain fog’. She articulated
a perceived improvement in her overall quality of life, citing
increased physical activity, which included a resumption of
gym visits. The patient’s testimony corroborates the quantifiable data, encapsulating a recovery trajectory that restored her
functional capabilities to a level not experienced in the three
years preceding the intervention.
Discussion
Compartment Syndrome is a complex pathological state
where increased intracompartmental pressure, primarily in
the lower extremities, can precipitate a cascade of detrimental
events ranging from ischemic injury to irreversible tissue and
nerve damage. This syndrome, influenced by a spectrum of
etiologies from traumatic injuries to repetitive exertional activities, presents unique clinical dilemmas, especially in its chronic
form which often resists standard treatment modalities [7].
While the acute version of this syndrome requires surgical action, the chronic type, can be subtle and frequently resistant to
conventional treatments, as highlighted in our case.
The transition from acute to chronic stages of compartment
syndrome particularly complicates management strategies, as
the long-term sequelae of sustained intra-compartmental pressure may not be as immediately apparent or responsive to conventional surgical interventions like fasciotomy. In the chronic
variant, known as Chronic Exertional Compartment Syndrome
(CECS), patients may experience a recurring or persistent symptomatology that subtly undermines their quality of life and
physical functionality [15].
The presented case of a 31-year-old female illustrates the
typical progression of compartment syndrome, exacerbated by
increased physical activity, and unresponsive to conventional
therapies including conservative management and fasciotomy.
The patient’s protracted journey, marked by recurrent pain
and functional limitation, underscores the intricate nature of
compartment syndrome and the challenges it poses in terms
of accurate diagnosis, effective management, and the pursuit
of lasting relief. In this case, the initial conservative approach,
including physical therapy and conservative management of
stress fractures, failed to provide lasting relief. Bilateral fasciotomies with revisions yielded only temporary symptom abatement, with the patient experiencing a symptomatic resurgence
in her left lower extremity.
In this context, SCS, traditionally reserved for neuropathic
pain syndromes, emerges as a novel and promising alternative
for compartment syndrome. Our patient’s significant and persistent pain relief following SCS implantation exemplifies the
device’s capacity to modulate aberrant neural pathways and
alter pain perception. This neuromodulatory technique offers
a non-pharmacological, reversible approach to pain management, distinct from the irreversible nature of surgical fasciotomy, which may entail complications and a risk of morbidity from
repeated interventions [18].
The implementation of SCS in compartment represents a
groundbreaking application of this technology, broadening its
use to encompass refractory musculoskeletal conditions. The
favorable outcome in our patient’s case, with a sustained reduction in pain of >90%, suggests that SCS may be a viable component in a multidisciplinary treatment strategy for compartment
syndrome, offering an alternative for patients who have not
benefited from traditional treatments. The reduction in pain
and improvement in functional outcomes associated with SCS
may decrease the reliance on surgical procedures and pharmacologic intervention, which, while sometimes necessary, do not
always offer a sustainable solution and have high abuse potential. While this case highlights the therapeutic potential of SCS
in chronic exertional compartment syndrome, marking a paradigm shift in treatment approaches, it’s important to acknowledge that there is a paucity of research and case reports on this
topic and that the field would benefit from more case reports
and Randomized Control Trials (RTC). The interdisciplinary nature of this intervention, integrating medical, surgical, and technological expertise, represents a new phase in patient-centric
care that prioritizes individualized treatment plans and aims for
superior clinical outcomes.
The application of SCS in the treatment of compartment
syndrome illustrates the expanding capabilities of neuromodulation in managing complex pain syndromes. It reinforces the
importance of considering innovative, interdisciplinary approaches when conventional treatments fail, paving the way for
patient-specific strategies that hold the promise of improved
life quality and functional recovery. The advancement of SCS
has expanded their clinical applications, as evident from our
case study underscores the potential of interdisciplinary collaborations in enhancing treatment outcomes. While surgical fasciotomy is a mainstay for compartment syndrome, neuromodulatory techniques like the SCS present promising alternative
strategies. As the trajectory of medical therapeutics continues
to evolve, such interdisciplinary collaborations offer a new era
of patient care, characterized by individualized treatments and
enhanced patient outcomes.
Declarations
Data availability statement: Data sharing is not applicable
to this article as no new data were created or analyzed in this
study.
Author contributions: JD and KA responsible for manuscript
preparation, writing, and editing MP and NT were responsible
for overseeing case and manuscript.
Funding: No funding was acquired during this case.
Ethical approval: Authors adhered to the reporting guidelines as outlined by CARE Guidelines and completed the corresponding CARE Checklist.
Competing interests: The authors declare no competing interests.
References
- Shinu P, Morsy MA, Nair AB, Mouslem AKA, Venugopala KN, et al. Novel Therapies for the Treatment of Neuropathic Pain: Potential and Pitfalls. J Clin Med. 2022; 11(11): 3002.
- Van Buyten JP. Neurostimulation for Chronic Neuropathic Back Pain in Failed Back Surgery Syndrome. J Pain Symptom Manage. 2006; 31(4): S25-9.
- North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal Cord Stimulation versus Repeated Lumbosacral Spine Surgery for Chronic Pain: A Randomized, Controlled Trial. Neurosurgery. 2005; 56(1): 98-107.
- Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: A multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007; 132(1): 179-88.
- Taylor RS. Spinal Cord Stimulation in Complex Regional Pain Syndrome and Refractory Neuropathic Back and Leg Pain/Failed Back Surgery Syndrome: Results of a Systematic Review and Meta-Analysis. J Pain Symptom Manage. 2006; 31(4): S13-9.
- Kemler MA, De Vet HCW, Barendse GAM, Van Den Wildenberg FAJM, Van Kleef M. The effect of spinal cord stimulation in patients with chronic reflex sympathetic dystrophy: Two years’ follow‐up of the randomized controlled trial. Ann Neurol. 2004; 55(1): 13-8.
- Gersbach P, Hasdemir MG, Stevens RD, Nachbur B, Mahler F. Discriminative microcirculatory screening of patients with refractory limb ischaemia for dorsal column stimulation. Eur J Vasc Endovasc Surg. 1997; 13(5): 464-71.
- Amann W, Berg P, Gersbach P, Gamain J, Raphael JH, et al. Spinal cord stimulation in the treatment of non-reconstructable stable critical leg ischaemia: Results of the European Peripheral Vascular Disease Outcome Study (SCS-EPOS). Eur J Vasc Endovasc Surg. 2003; 26(3): 280-6.
- Sdrulla AD, Guan Y, Raja SN. Spinal Cord Stimulation: Clinical Efficacy and Potential Mechanisms. Pain Pract. 2018; 18(8): 1048-67.
- Caylor J, Reddy R, Yin S, Cui C, Huang M, et al. Spinal cord stimulation in chronic pain: Evidence and theory for mechanisms of action. Bioelectron Med. 2019; 5(1): 12.
- Jeon YH. Spinal Cord Stimulation in Pain Management: A Review. Korean J Pain. 2012; 25(3):143-50.
- Guzzi G, Della Torre A, La Torre D, Volpentesta G, Stroscio CA, et al. Spinal Cord Stimulation in Chronic Low Back Pain Syndrome: Mechanisms of Modulation, Technical Features and Clinical Application. Healthcare. 2022; 10(10): 1953.
- Wolter T. Spinal cord stimulation for neuropathic pain: Current perspectives. J Pain Res. 2014; 651.
- Yu K, Niu X, He B. Neuromodulation Management of Chronic Neuropathic Pain in the Central Nervous System. Adv Funct Mater. 2020; 30(37): 1908999.
- Chandwani D, Varacallo M. Exertional Compartment Syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing. 2023. http://www.ncbi.nlm.nih.gov/books/NBK544284/.
- Torlincasi AM, Lopez RA, Waseem M. Acute Compartment Syndrome. In: StatPearls Treasure Island (FL): StatPearls Publishing. 2023. http://www.ncbi.nlm.nih.gov/books/NBK448124/.
- Pearse MF. Acute compartment syndrome of the leg. BMJ. 2002; 325(7364): 557-8.
- Mangan JJ, Rogero RG, Fuchs DJ, Raikin SM. Predictors of Improvement After Fasciotomy for Treatment of Chronic Exertional Compartment Syndrome of the Lower Extremity. Sports Health Multidiscip Approach. 2021; 13(4): 396-401.