Introduction
Autism Spectrum Disorder (ASD) is a complex neurodevelopmental disorder with early onset, characterized by two core
symptoms domains: social communication and repetitive behaviours [1]. ASD is a multi-factorial disorder in nature with
genetic mechanisms, immune dysregulations and neurofunctional alterations as pathogenic mechanisms [2]. First, specific genetic syndromes like Rett syndrome or Fragile-X syndrome,
or cytogenetic abnormalities are associated with ASD. Moreover,
specific mutations have been identified in ASD [3]. SCN2A,
encoding the neuronal voltage-gated Na+ channel NaV1.2,
is one of the most commonly affected loci linked to Autism
Spectrum Disorders (ASDs) [4]. Second, strong inflammation
states are associated with ASD, including increased levels of
cytokines [5]. Moreover, an excessive microglial activation (related to immune abnormalities) has been observed in multiple
brain regions of people with ASD [6]. Third, altered functional and structural organization of the Default Mode Network
(DMN) are prominent neurobiological features of ASD, as well
as a shallower right TPJ sulcus, a thicker mPFC and abnormal cingulate cortex [7]. In conjunction with behavioural and
emotional therapies [8], pharmacological approach is often
considered [9,10]. Medications such as risperidone and aripiprazole have an effect on ASD related irritability and
aggression [11], with risperidone being the only one drug approved by the FDA to treat the irritability symptoms [12]. However, there are no established pharmacological treatment
for the core features of ASD.
Nowadays, there is still increasing the interest is non-invasive brain stimulation in the treatment of neurodevelopmental disorders, thanks to their ability to modulate neuroplasticity, behavioral and socio-emotional processes [13,14].
One of the new neuromodulation approaches is the transcranial photobiomodulation (tPBM), In PBM there is a noninvasive exposure of light, typically within the red to nearinfrared (~λ=600-1300 nm) spectrum, to elicit physiological
effects across several tissue systems [15]. PBM can include
either coherent-light (lasers) or non-coherent light (lightemitting diodes, LEDs) [15] and its therapeutic mechanisms involved increased metabolism, plastic modulation of neural
networks and anti-inflammatory effects [16].
Considering the clinical phenotype of ASD, transcranial
PBM (tPBM) appears as a potential optimal candidate for
its treatment. A study with a mouse model of Valproic- Acid
(VPA)-induced autism showed that laser PBM with a wavelength of 830 nm as a neuroprotective effect, inducing an
attenuation of cognitive dysfunction, repetitive behaviours
and neuroinflammation [17]. Specifically, a redunction in the
number of microglia in hippocampus was reported. The first
study with PBM in human with ASD employed low-level laser
therapy, which is a form of PBM, with a pulsed laser of 635 nm
with a power output of 15 mW. Laser was delivered to the
base of the skull and temporal areas children and adolecents.
A reduction in the aberrant behaviours and an improvement
in the clinical global impression scale were observed [18].
The first study adopting LED PBM was published by our group
[19]. LED light has the advantage of irradiating larger areas
of tissue and results in fewer side effects. 21 patients with a
mean age of 9.1 were treated with LED PBM at 810 nm 5 days
a week for 6 months. The protocol involves two sessions a day:
one with light delivered at 10 Hz (alpha stimulation) and one at
40 Hz (Gamma stimulation). The main result was the reduction
in ASD severity. Secondary measures showed a reduction in
cognitive and behavioral rigidity and an improvement in
parents-reported attention. Another study showed the effects
of tPBM at a wavelength of 830 nm in adults with high- functioning ASD [20], reporting a reduction in the severity of ASD symptoms. All these findings converge to report that tPBM is a safe and
feasible treatment approach for ASD. In this single study, we report the case of a young adult with gene SCN2-A-related autism
and hypofrontality, which was treated with tPBM and showed
unbelievable outcomes.
Methods
Case description
All the clinical data of the patient were extracted from databases containing information on patients of the psychiatric
clinic at the Institute of Neuroscience, Florence (Italy).
The patient, aged 24, presented a clinical picture characterised by difficulties in social interaction, generalised and performance anxiety, difficulty in modulating mood and easy irritability and difficulty in accepting changes.
In 2009, the first psychopathological assessments were
carried out in which he was diagnosed with ASD. Moreover,
the tendency to rigid submission to daily routines and habits
was observed as well as a phantasmatic personality structure,
explored through projective tests.
In July 2010, another psychopathological assessment was
carried out in which an important generalised anxiety disorder
was reported, associated with somatisation, grandiose ideas
and accelerated speech. Oppositional defiant traits with occasional rule- breaking and impulsiveness were also highlighted. A
highly immature personality structure also emerged, with a disharmonious profile, difficulties in emotional modulation, poor
self-control and recourse to poorly evolving and dysfunctional
defence mechanisms, such as switching to behavioural action in
the face of frustration.
Following the assessment, a weekly psychotherapeutic course
was undertaken, which was subsequently interrupted by the
During the high school, parents and teachers reported good
commitment and motivation in the school environment as well
as an accentuation of anxiety reactions in the face of both
scholastic and performance demands and a greater awareness
of his difficulties, with a tendency to become anxious and disorganised even in the face of small mishaps and mistakes in daily
life. They report, therefore, a rigidity in coping with the rhythms
of daily life, presenting difficulties and sometimes resisting
changing habits and needing ample notice of any changes.
They also report extreme attention to schedules with significant anticipatory agitation in the face of appointments with
related disorganised beahviours and anxiety. A scarse ability
of self-regulation was observed. There was a permanent difficulty in interacting with the peer group. He also reported difficulty in managing his impulses, aggressive behaviours.
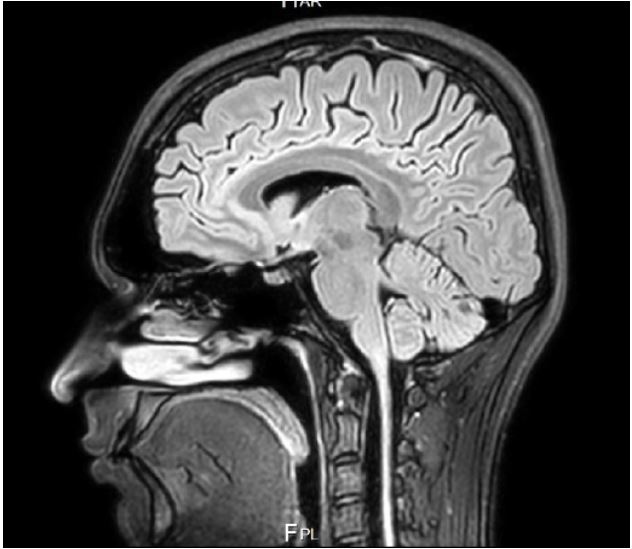
In 2020, structural and functional MRI showed hypofrontalily, i.e. decreased neural activity in the frontal regions, an
abnormal paracingular sulcus and a lower than normal position
of the cerebellar tonsils at the level of the great occipital foramen (Figure 1).
Furthermore, he reported altered values in the Reuma test,
meaning an ongoing inflammatory process. When a genetic test
was conducted, a mutation in gene SCN2A was observed.
In July 2022, he was admitted to the outpatient psychiatric unit at the Institute of Neuroscience.
He received a diagnosis of ASD with genetic typing (SNC2A). He was assessed with psychometric and neuropsychological measures. The Ritvo Autism Asperger Diagnostic ScaleRevised (RAADS-R) confirmed the presence of autistic traits.
The Montefiore- Einstein Rigidity Scale (MERS) showed increased levels of cognitive and behavioural rigidity along with
severe protest. The Reactivity, Intensity, Polarity and Stability questionnaire (RIPoSt-40) highlights emotional dysregulation and liability. Specifically, neuropsychological assessment
included Connor’s Continuous Performance Task (CPT) and Stop
Signal Task, showing control inhibition deficits.
In this case, PBM was considered and added to ongoing
behavioral or pharmacological treatments, which remained
unchanged throughout the stimulation period. Pharmacological therapy included Lorazepam (2 mg/die) and atomoxetine.
Procedure and stimulation parameters
tPBM was delivered using the commercially available
Vielight®Neuro Gamma brain photo biomodulation stimulator.
The gamma stimulator pulses light at 40 Hz light pulsing frequency and delivers 810 nm near infrared light via the transcranial LED
clusters placed on the helmet.
The device is composed of a wearable headset with features
microchip-boosted transcranial LED diodes. The tPBM headset
consists of four clusters. The four LEDs deliver the NIR to
the subdivisions of the DMN: The medial prefrontal cortex,
the praecuneus area, and left and right angular gyrus. The
intranasal application is positioned in the left nostril with the
clip on the outside to deliver light to the ventral section of the
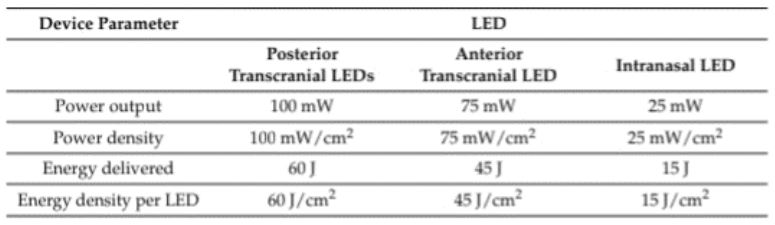
brain, specifically to the ventromedial PFC. Device parameters
are reported in Figure 2. tPBM was applied daily (5 days a week)
for a total of 60 sessions.
Results
tPBM was well tolerated and no side effects were observed.
At the end of treatment, an improvement in ASD core features
was observed (Table 1). Indeed, standardized metrics reported a reduction in repetive behaviours, measured through
the The Adult Repetitive Behaviours Questionnaire-2 (RBQ2), an improvement in impulsivity, measured through the
UPPS-P Impulsive Behavior Scale, a reduction in cognitive and
behavioural rigidity, measured through the MERS, and an
improvement in empathy, assessed with the Empathy Quotient (EQ). Furthermore, an improvement in emotion liability,
measured with the RiPOST was observed. Importantly, tPBM
was associated with a significant reduction in related disability (SDS). At a 12-month follow-up improvement was
stable.
Moreover, tPBM improved executive and attentional functions, as measured with the CPT and Stop Signal Task. Specifically, a profile of attentional function with a 75% of probability
of being clinical reduced to a probability of 50%. Moreover, an
increase in reaction time (T0:210 ms; T1:240 ms) was observed
in SST, meaning an improvement in impulsivity.
Discussion
The presented case study of a young adult with SCN2A-related autism further highlights the potential of tPBM as a therapeutic option in ASD. Specifically, tPBM resulted to decrease
ASD symptoms severity, like repetitive behaviours, impulsive
behaviours, and behavioural rigidity. Moreover, executive and
attentional functions improved. Indeed, the Stop Signal Reaction
Time (SSRT) increased after the treatment, meaning an attenuation of impulsive behaviours. Improvement in ASD severity
here reported is consistent with previous findings [18-20]. Also
the improvement in attention was previously reported by [19].
This is the first time that an improvement in impulsive behaviours, corroborated by a neuropsychological test, is shown in
ASD following tPBM. Importantly, no previous study reported
whether tPBM could be effective in ASD with specific underlying
genetic mutations and important structural and functional MRI
abnormalities.
Table 1: Psychometric test scores at the baseline, at the end of
the treatment (after two months) and at a 12-month follow-up.
| Test name |
Pre-Test |
Post-Test |
Difference |
Follow-up-Test |
| RBQ-2 |
1.93 |
1.35 |
-0.58 |
1.27 |
| UPPS-P |
71 |
34 |
-37 |
32 |
| MERS |
22 |
15 |
-7 |
16 |
| EQ |
29 |
32 |
+3 |
34 |
| RIPOST |
194 |
165 |
-29 |
139 |
| SDS |
26 |
5 |
-21 |
5 |
SCN2A, encoding the NaV1.2 sodium channel, is one of the
commonly affected loci in ASD but also in epilepsy and infantile
seizures [21]. In SCN2A-related epilepsy, mutations resulted in a
gain of function which led to an increased neural excitability [21].
In children with SCN2A-related disorders the pathogenic variant
(“mutation”) in the gene SCN2A is associated with a dampened
channel function, which impair the flow of sodium ions in the
brain, leading to deficits in neural excitability [21]. This could explain the hypofrontality observed in our patient. Moreover, the
loss of sodium reduced action potential backpropagation into
dendrites, impairing synaptic plasticity and synaptic strength
[22]. Interestingly, PBM has been showed to increase the number of dendritic nodes and ends [23], restoring dendritic functioning [24], and improve synaptic plasticity [25]. Furthermore, a
potential mechanism of tPBM is associated with cerebrovascular
oxygenation of the prefrontal cortex [26]. Moreover, a systematic review showed strong support for long-range underconnectivity in ASD and that ASD is characterized by a general trend
toward an under-expression of lower-band wide-spread integrative processes compensated by more focal, higher-frequency, locally specialized, and segregated processes [27].
tPBM at 40 Hz to the Default Mode Network (DMN) significantly increases the power of the higher oscillatory frequencies
of alpha, beta and gamma and reduces the power of the slower
frequencies of delta and theta in subjects in resting state [28].
Therefore, through a precision approach [29], tPBM could
restore neural oscillations and altered brain wave patters
observed in ASD, leading to an improvement in ASD
symptomatology.
Remarkably, the neural networks restorations should be
stable in time. Indeed, in SCN2A-related ASD, the pathogenic
mechanism leading to alterations is active only in early brain
development, because in the mature brain NaV1.6 replaced
NaV1.2 [21]. Coherently, herein we reported the sustained improvements at a 12-month follow-up, a finding that reinforces
the potential durability of tPBM effects. These findings align
with other studies reporting the benefits of tPBM in individuals
with ASD [20]. Collectively, the evidence converges to suggest that tPBM is a safe and feasible therapeutic option for individuals with SCN2A-related ASD, offering potential improvements
in core symptoms and associated challenges.
Furthermore, a gastrointestinal dysfunction has been identifying in genetically determined ASD cases [30]. Indeed, a disruption of the gut-brain axis has been implicated in ASD and correlated with brain gene expression changes, restrictive dietary
patterns and pro-inflammatory cytokine profiles [31]. In this
msense, PBM has been shown to positively affect and control
microbiome [32] and to regulate cytokines levels [33]. One interesting option, even if premature but to be explored is represented by the potential application of this treatment to other
phenotypical SCN2A-related disorders include, seizures.
Conclusion
In conclusion, the present case study contributes to the
growing body of literature supporting tPBM as a novel approach
for individuals with SCN2-A related ASD. The combination of
genetic susceptibility, immune dysregulations, and neurofunctional alterations in ASD makes tPBM a compelling intervention.
While further research is needed to elucidate the underlying
mechanisms and optimize treatment protocols, the potential
of tPBM to modulate neural activity and positively impact core
ASD features is promising. As the field advances, tPBM may
emerge as a valuable addition to the toolkit of interventions for
individuals with ASD, offering hope for improved quality of life
and socio-emotional functioning.
Acknowledgement
Informed consent: The patient gave his informed consent for
the inclusion of his data in this case report.
Data avaiablity statement: The data that support the
findings of this study are available on request from the corresponding author. The data are not publicly available because
containing information that could compromise the privacy of
research participants.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed,textrev.). 2022. https://doi.org/10.1176/appi.books.9780890425787.
- Meltzer A, Van de Water J. The Role of the Immune System in Autism Spectrum Disorder. Neuropsychopharmacology: Official publication of the American College of Neuropsychopharmacology. 2017; 42(1): 284-298. https://doi.org/10.1038/npp.2016.158.
- Geschwind DH. Genetics of autism spectrum disorders. Trends in cognitive sciences. 2011; 15(9): 409-416.
- Wang HG, Bavley CC, Li A, Jones RM, Hackett J, et al. Scn2a severehypomorphic mutation decreases excitatory synaptic input and causes autism-associated behaviors. JCI insight. 2021; 6(15).
- Siniscalco D, Schultz S, Brigida AL, Antonucci N. Inflammation and neuro-immune dysregulations in autism spectrum disorders. Pharmaceuticals. 2018; 11(2): 56.
- Suzuki K, Sugihara G, Ouchi Y, Nakamura K, Futatsubashi M, et al. Microglial activation in young adults with autism spectrum disorder. JAMA psychiatry. 2013; 70(1), 49-58.
- Padmanabhan A, Lynch CJ, Schaer M, Menon V. The default mode network in autism. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2017; 2(6): 476-486.
- Trembath D, Varcin K, Waddington H, Sulek R, Bent C, et al. Nonpharmacological interventions for autistic children: An umbrella review. Autism. 2023; 27(2): 275-295.
- Eissa N, Al-Houqani M, Sadeq A, Ojha SK, Sasse A, et al. Current enlightenment about etiology and pharmacological treatment of autism spectrum disorder. Frontiers in neuroscience. 2018; 12: 304.
- Pallanti S, Bencini L, Cantisani A, Hollander E. Psychotropic treatment of autism. Autism Spectrum Disorders. 2015; 180: 151-165.
- DeVane CL, Charles JM, Abramson RK, Williams JE, Carpenter LA, et al. Of autism spectrum disorder: results from the randomized BAART clinical trial. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2019; 39(6): 626-635.
- Sharma SR, Gonda X, Tarazi FI. Autism spectrum disorder: Classification, diagnosis and therapy. Pharmacology & therapeutics. 2018; 190: 91-104.
- Finisguerra A, Borgatti R, Urgesi C. Non-invasive brain stimulation for the rehabilitation of children and adolescents with neurodevelopmental disorders: A systematic review. Frontiers in psychology. 2019; 10: 135.
- Enticott PG, Pallanti S, Hollander E. Transcranial magnetic resonance and noninvasive brain stimulation. In Autism Spectrum Disorders; Hollander, E,Hagerman, R.J,Fein, D,Eds.; American Psychiatric Association Publishing: Washington DC, USA. 2018.
- Hamblin MR. Photobiomodulation or low-level laser therapy. Journal of biophotonics. 2016; 9(11-12): 1122.
- de Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE Journal of selected topics in quantum electronics. 2016; 22(3): 348-364.
- Kim UJ, Hong N, Ahn JC. Photobiomodulation attenuated cognitive dysfunction and neuroinflammation in a prenatal valproic acid-induced autism spectrum disorder mouse model. International Journal of Molecular Sciences. 2022; 23(24): 16099.
- Leisman G, Machado C, Machado Y, Chinchilla-Acosta M. Effects of low-level laser therapy in autism spectrum disorder. Clinical Medicine Research. 2018; 111-130.
- Pallanti S, Di Ponzio M, Grassi E, Vannini G, Cauli G. Transcranial photobiomodulation for the treatment of children with autism spectrum disorder (ASD): A retrospective study. Children. 2022; 9(5): 755.
- Ceranoglu TA, Cassano P, Hoskova B, Green A, Dallenbach N, et al. Transcranial photobiomodulation in adults with highfunctioning autism spectrum disorder: Positive findings from a proof-of-concept study. Photobiomodulation, Photomedicine, and Laser Surgery. 2022; 40(1): 4-12.
- Ben-Shalom R, Keeshe CM, Berrios KN, An JY, Sanders SJ, et al. Opposing effects onNaV1. 2 function underlie differences between SCN2A variants observed in individuals with autism spectrum disorder or infantile seizures. Biological psychiatry. 2017; 82(3): 224-232.
- Spratt PW, Ben-Shalom R, Keeshen CM, Burke KJ, Clarkson RL, et al. The autism-associated gene Scn2a contributes to dendritic excitability and synaptic function in the prefrontal cortex. Neuron. 2019; 103(4): 673-685.
- dos Santos Cardoso F, Serra FT, Coimbra NC, Gonzalez-Lima F, da Silva SG. Transcranial photobiomodulation changes neuronal morphology in the cerebral cortex of rats. Neuroscience Letters. 2022; 781: 136681.
- Meng C, He Z, Xing D. Low-level laser therapy rescues dendrite atrophy via upregulating BDNF expression: implications for Alzheimer’s disease. Journal of Neuroscience. 2013; 33(33): 13505-13517.
- Buendía D, Guncay T, Oyanedel M, Lemus M, Weinstein A, et al. The transcranial light therapy improves synaptic plasticity in the Alzheimer’s disease mouse model. Brain Sciences. 2022; 12(10): 1272.
- Holmes E, Barrett DW, Saucedo CL, O’Connor P, Liu H, et al. Cognitive enhancement by transcranial photobiomodulation is associated with cerebrovascular oxygenation of the prefrontal cortex. Frontiers in Neuroscience. 2019; 13: 1129.
- O’Reilly C, Lewis JD, Elsabbagh M. Is functional brain connectivity atypical in autism? A systematic review of EEG and MEG studies. PloS one. 2017; 12(5): e0175870.
- Jahan A, Nazari MA, Mahmoudi J, Salehpour F, Salimi MM. Transcranial near-infrared photobiomodulation could modulate brain electrophysiological features and attentional performance in healthy young adults. Lasers in Medical Science. 2019; 34: 1193-1200.
- Liebert A, Capon W, Pang V, Vila D, Bicknell B, et al. Photophysical Mechanisms of Photobiomodulation Therapy as Precision Medicine. Biomedicines. 2023; 11(2): 237.
- Davidson EA, Holingue C, Jimenez-Gomez A, Dallman JE, Moshiree B. Gastrointestinal Dysfunction in Genetically Defined Neurodevelopmental Disorders. In Seminars in Neurology. 333 Seventh Avenue, 18th Floor, New York, NY 10001, USA: Thieme Medical Publishers, Inc. 2023.
- Morton JT, Jin DM, Mills RH, Shao Y, Rahman G, et al. Multi-level analysis of the gut–brain axis shows autism spectrum disorderassociated molecular and microbial profiles. Nature Neuroscience. 2023; 1-10.
- Bicknell B, Liebert A, Borody T, Herkes G, McLachlan C, et al. Neurodegenerative and Neurodevelopmental Diseases and the Gut-Brain Axis: The Potential of Therapeutic Targeting of the Microbiome. International Journal of Molecular Sciences. 2023; 24(11): 9577.
- Shamloo S, Defensor E, Ciari P, Ogawa G, Vidano L, et al. The anti-inflammatory effects of photobiomodulation are mediated by cytokines: Evidence from a mouse model of inflammation. Frontiers in Neuroscience. 2023; 17: 1150156.