Introduction
A history of recurrent gastric or duodenal ulcers could indicate a gastrinoma, also known as Zollinger-Ellison Syndrome
(ZES). A gastrinoma is a neuroendocrine tumor that causes
inappropriate gastrin secretion, ultimately causing gastric acid
hypersecretion. This leads to symptoms of acid reflux, gastric
and duodenal ulcers, and anemia. For a diagnosis of ZES to be
confirmed, there must be evidence of hypersecretion of gastrin
and a decreased gastric pH. A serum gastrin level >1000 pg/mL
and a gastric pH of 2 are considered to be diagnostic for gastrinoma. Specific studies such as NG tube fluid aspiration analysis
or upper GI endoscopy can be used to measure the pH of the
stomach. CT, MRI, or endoscopic ultrasound scans can then be
used to localize the primary tumor. It should be noted that CT
scans are not sensitive to small liver lesions, so MRI is a better
option if metastatic disease is suspected [1]. Another technique
that is used to localize gastrinomas is the octreotide scan or somatostatin receptor scintigraphy. This involves the administration of radio-labeled octreotide, which has selective binding to
somatostatin receptors found on gastrinoma tumor cells. It has
Annals of Surgical
Case Reports & Images
high sensitivity and specificity for primary tumor detection and
metastatic lesions. Combined with single-photo emission CT,
this has shown an even higher sensitivity and specificity than CT
or octreotide scan imaging alone.
Gastrinomas are located mostly in the gastrinoma triangle;
the superior border of the triangle is the confluence of the cystic and common bile ducts, the inferior border is the second and
third portions of the duodenum, and the medial border is the
neck of the pancreas [2]. In the majority of cases, pancreatic
lesions have worse prognoses than duodenal lesions and are
more often associated with metastatic disease. About 25-33%
of patients who have gastrinomas develop them secondary to a
syndrome called Multiple Endocrine Neoplasia Type 1 (MEN-1)
syndrome [3]. This condition involves tumors of the parathyroid
gland, pituitary gland, and pancreas. Management of sporadic
gastrinomas compared to those associated with MEN-1 syndrome varies slightly, so it is important to make the distinction
between the two during the initial diagnosis. In patients with
MEN-1, in addition to symptoms of gastrin hypersecretion,
there would be signs of hyperparathyroidism, such as hypercalcemia, and symptoms of a pituitary adenoma. MEN-1 syndrome with hyperparathyroidism leads to increased serum calcium, a known stimulator of gastrin release from gastrinomas.
Because of this, patients with MEN-1 syndrome have increased
resistance to anti-secretory medical treatments and require different doses of treatment drugs than patients who have sporadic ZES [4]. Another difference between MEN-1-related gastrinomas and sporadic gastrinomas is that the former is usually
associated with duodenal lesions, while the latter lesions usually localize in the pancreas. Sporadic pancreatic gastrinomas
metastasize to regional lymph nodes in approximately 60% of
patients, and to the liver in 10-20% of patients, a much higher
frequency than that of duodenal gastrinomas. The 10-year survival rate of those patients with sporadic disease is therefore
much lower than in patients with duodenal gastrinomas [3].
Symptomatic treatment starts with Proton Pump Inhibitors
(PPIs), however, in cases where the gastrinomas metastasize,
PPIs alone are not adequate for relief. Octreotide injections
can also stabilize gastrin secretion symptoms, thereby reducing
symptoms, but surgery is the only curative treatment to this day.
Surgery is useful in controlling the increased secretion of acid by
directly removing the secreting cells of gastrin and any metastases. If the metastases are unresectable, however, resecting the
primary tumor is still beneficial as long as 80-90% of the mass
can be safely removed [5]. After the procedure, it is important
to monitor gastrin and chromogranin A, a neuroendocrine biomarker, levels to check for recurrence or incomplete removal of
the disease. In this case report, we present a 59-year-old female
with a history of recurrent duodenal ulcers only to be found to
have a pancreatic gastrinoma with extensive liver metastasis.
Gastrinomas with liver metastasis are associated with significantly reduced survival, therefore our patient’s best chance of
improving her survival rate was to undergo aggressive resection
of the tumor and its associated liver metastases.
Case presentation
A 59-year-old female with a past medical history of hypertension, GERD, and a perforated duodenal ulcer presented to
the ED with epigastric pain, nausea, vomiting, and melena in
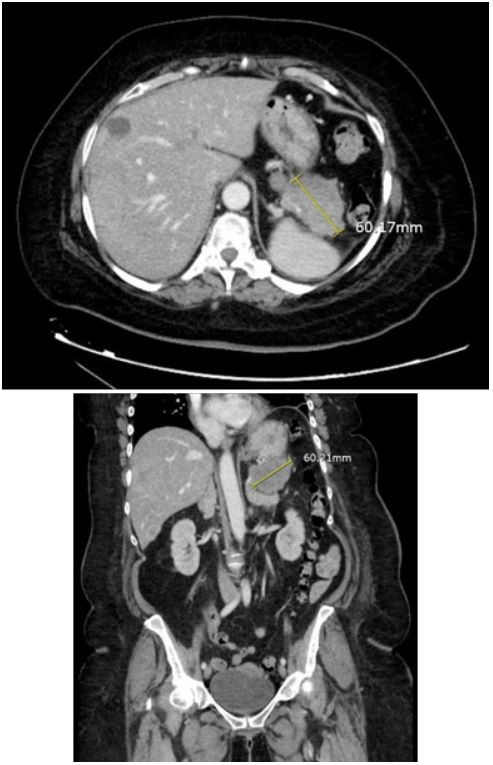
November of 2023. A CT scan of the chest, abdomen, and pelvis
showed a 6 cm pancreatic tail mass with numerous liver lesions
(Figure 1). The patient continued to pass dark stools throughout her hospital stay. An EGD done a few days later showed a
non-bleeding 2 cm duodenal ulcer. The ulcer was treated with
bipolar cautery. The second, third, and fourth portions of the
duodenum were normal. H. pylori testing came back negative.
Her EGD done in 2022 also showed evidence of gastritis, esophagitis, and two ulcers in the duodenum, indicating a chronic history of peptic ulcer disease. The patient, however, continued to
have symptomatic anemia and pass dark stools. A mesenteric
angiogram with gastroduodenal artery embolization was done,
and the patient noted relief of melena; she was discharged to
follow up as an outpatient. 5 days post-discharge, the patient
came back to the ED presenting with weakness, inability to ambulate, and melena as of that morning. She was initially hypotensive but this improved with fluids. Her CEA, CA 19-9 and AFP
were negative. Studies revealed gastrin levels to be 2745 pg/
mL, raising concern for her pancreatic mass to be a gastrinoma.
MRI showed a 5.0 mass in the tail of the pancreas, and multiple
masses within the liver measuring up to 6.0 cm, confirming the
diagnosis of a gastrinoma with liver metastases. She was then
started on octreotide three times a day. The patient was scheduled for a distal pancreatectomy and liver metastases. Platelets and hemoglobin remained stable, so the patient was taken for
surgery. During the procedure, the abdomen was inspected and
no diffuse carcinomatosis was found. The tumor at the distal
pancreas was visualized. The pancreas was dissected off the
retroperitoneum. The tumor was taken out and sent for a frozen section and came back with negative margins. 12 hepatic
tumor metastases were resected. The procedure was complicated by bleeding from a previously diagnosed duodenal ulcer.
The source of the bleeding was ligated after making a duodenotomy and suturing the bleeders using a three-point ligation
technique.
Discussion
With our patient’s history of peptic ulcer disease, her symptoms when she initially presented to the ED, and her increased
gastrin levels, the patient had a classic presentation of pancreatic gastrinoma. Imaging confirmed the diagnosis and the
presence of liver metastasis, and after a multidisciplinary board
discussion, the decision was made to proceed with surgical resection.
In cases that are associated with MEN-1 syndrome, diagnosis of the presence of a gastrinoma is not as straightforward as
many patients with MEN-1 only show symptoms of ZES after
the development of hyperparathyroidism. Hypercalcemia secondary to hyperparathyroidism can be the first sign of MEN-1,
and it is directly associated with the later development of hypergastrinemia [6]. As it stands today, surgery is the only curative management for gastrinoma with liver metastasis. PPIs and
H2 blockers are considered for symptomatic management during the early stages of the disease, when the gastrinoma and/or
metastases are not resectable, or in cases where there is widespread metastasis.
Hepatic and/or gastroduodenal artery embolization is another therapeutic option to reduce metastatic symptoms as well as
control peptic ulcer symptoms. Liver metastases get their blood supply from the hepatic artery, so embolization of the hepatic
artery can reduce symptoms of the metastases. One must factor in the possibility of postembolization syndrome, however,
which can present as fever, nausea, loss of appetite, and abdominal pain. However, in cases as severe as this one, such a
risk does not outweigh the benefits of the procedure [7].
As true with most metastatic diseases, the treatment options
are limited. Chemotherapy is an option for patients with distant
metastases, however, this treatment method has limited effects
on disease regression and can prove to be more toxic than helpful to the patient. Studies also show that octreotide administration can control the growth of liver metastases and stabilize
serum gastrin secretion, but this will not rid the patient of the
disease itself. Shanshank et al. 2023 state that targeted therapies such as antiangiogenic modalities, multi-kinase, or mTOR
inhibition are upcoming new approaches to tackling delayed
tumor progression in patients with metastatic disease. These
novel therapies are still in the trial phase, however, so the most
effective treatment for gastrinoma is still surgical resection.
Lastly, orthotopic liver transplantation is currently being explored as an option for patients who have limited liver metastatic resectibility. The survival rate is similar to that of hepatic
resection, however, this needs to be studied further before it
can be a definitive option for such patients [8].
The extent and location of metastases are the most important determining factors for mortality. For metastatic disease,
removal of the primary tumor and as many metastases as possible is the goal to prevent further spread of the disease and
improve survival. As our patient did have extensive liver metastasis, there is a possibility that the disease has not been completely eradicated. Good post-operative care and consistent
follow-up are key for this patient; any sign of lingering disease
or recurrence should be caught early and treated immediately.
Checking serum gastrin levels and chromogranin A regularly
post-surgery is vital to monitor the disease regression.
Conclusion
Recognizing that a patient has ZES can be a challenge within itself. It can take a long time to diagnose as the symptoms
initially appear as non-specific manifesting as abdominal pain,
chronic diarrhea, GERD, and anemia. In patients with chronic or
recurrent ulcers, the diagnosis of gastrinoma should be high on
the list of differentials. If applicable, symptomatic management
with PPIs and imaging would be the next steps to identify the
tumor and the extent of its metastases. Surgical resection remains to be the only curative treatment, however, with upcoming advances in medicine, it may become possible to rely more
on medical treatments to aid in the curative process of ZES with
liver metastases.
References
- Rossi RE, Elvevi A, Citterio D, Coppa J, Invernizzi P, et al. Gastrinoma and Zollinger Ellison syndrome: A roadmap for the management between new and old therapies. *World Journal of Gastroenterology. 2021; 27(35): 5890-5907. doi: 10.3748/wjg.v27.i35.5890
- Cingam SR, Botejue M, Hoilat, GJ, et al. Gastrinoma. In: StatPearls. 2023. https://www.ncbi.nlm.nih.gov/books/NBK441842/
- Anlauf M, Garbrecht N, Henopp T, Schmitt A, Schlenger R, et al. Sporadic versus hereditary gastrinomas of the duodenum and pancreas: distinct clinico-pathological and epidemiological features. World Journal of Gastroenterology. 2006; 12(34): 5440-5446. https://doi.org/10.3748/wjg.v12.i34.5440
- Norton JA, Foster DS, Ito T, Jensen RT. Gastrinomas: Medical or Surgical Treatment. Endocrinology and Metabolism Clinics of North America. 2019; 47(3): 577-601. doi: 10.1016/j.ecl.2018.04.009
- Zheng M, Li Y, Li T, Zhang L, Zhou L. Resection of the primary tumor improves survival in patients with gastro-entero-pancreatic neuroendocrine neoplasms with liver metastases: A SEER-based analysis. Cancer Medicine. 2019; 8(11): 5128-5136. https://doi.org/10.1002/cam4.2431
- Massironi S, Rossi RE, Laffusa A, Eller-Vainicher C, Cavalcoli F, et al. Sporadic and MEN1-related gastrinoma and Zollinger-Ellison syndrome: differences in clinical characteristics and survival outcomes. Journal of Endocrinological Investigatio. 2013; 46(5): 957-965. doi: 10.1007/s40618-022-01961-w
- Jilesen AP, Klümpen HJ, Busch OR, van Gulik TM, van Lienden KP, et al. Selective Arterial Embolization of Liver Metastases from Gastrinomas: A Single-Centre Experience. ISRN Hepatology. 2013; 174608. doi: 10.1155/2013/174608
- Shao QQ, Zhao BB, Dong LB, Cao HT, Wang WB. Surgical management of Zollinger-Ellison syndrome: Classical considerations and current controversies. World Journal of Gastroenterology. 2019; 25(32): 4673-4681. https://doi.org/10.3748/wjg.v25.i32.4673.