Introduction
A gastroesophageal junction mass can be indicative of a
malignant lesion. Gastric cancer can be deadly if not treated
quickly and effectively. Identifying the cancer at an early stage
is crucial to improving the rate of survival in affected patients;
at this point, there is no prophylactic screening in the United
States for gastric cancers. Diagnosis of gastric malignancies usually only occurs after patients are symptomatic, presenting with
manifestations such as abdominal pain, anemia, weight loss, or
because of an incidental finding on imaging. Histological classification of the gastric tumor cells via endoscopic biopsy is required to confirm the diagnosis of gastric cancer and specify the
type of lesion, such as gastric adenocarcinoma or Gastrointestinal Stromal Tumor (GIST). The overall survival of advanced gastric adenocarcinoma, once a diagnosis has been confirmed, is
only three to five months without any interventions; therefore,
treatment is essential. Surgical resection of the mass following
or followed by chemotherapy provides the most prolonged survival rate in patients with gastric cancer.
An innovative study called the Medical Research Council
Adjuvant Gastric Infusional Chemotherapy trial, also known as
the MAGIC trial, evaluated the effects of pre-operative chemotherapy on gastric, esophageal, or gastroesophageal junction
tumor regression. The study showed that the overall survival of
patients with adenocarcinoma of the stomach or esophagus is
significantly improved when compared to that of patients who
underwent surgery alone [1]. This trial set a precedent for a
new method of increasing the effectiveness of treating gastric
cancers. Chemotherapy on its own can also be palliative for patients who have unresectable or recurrent tumors [2].
There are three types of Gastroesophageal Junction (GEJ) tumors; the Mary-Seiwert classification of a GEJ mass is based on
the location of the epicenter of the tumors and its relationship
with the gastroesophageal junction. Type I lesions are located
5 cm proximal to the GEJ, type II lesions span the GEJ and have
their focal point up to 2 cm below the GEJ, and type III cardiac
tumors extend up to 5 cm into the stomach [3]. It is important
to differentiate between the three to have optimal surgical
planning and specified pre-operative and post-operative care [4]. One of the most common procedures for the removal of a
gastroesophageal mass is an Ivor Lewis esophagectomy, which
involves a laparotomy and a right thoracotomy for resection of
the tumor [5].
We present the case of a 58-year-old Hispanic male presenting with anemia, dizziness, and emesis, the plan was to do such
a procedure to resect a Type III GEJ gastroadenocarcinoma that
was spanning from the distal esophagus to the cardia of the
stomach using the Ivor Lewis esophagectomy technique. When
the mass was identified at the time of surgery, it was evident
that the tumor had already started invading the surrounding
structures, including the diaphragm and the aorta.
Case presentation
A 58-year-old male with a past medical history of hypertension, iron-deficiency anemia, and Type II diabetes mellitus presented to the ED in August of 2023 with dizziness, vomiting, and
anorexia. He also stated that he lost 7 lbs in the past 10 days. The
patient denies any significant family medical history. His initial
hemoglobin level on admission was 7.8. The patient continued
to bleed and required blood transfusions. A CT scan of his chest,
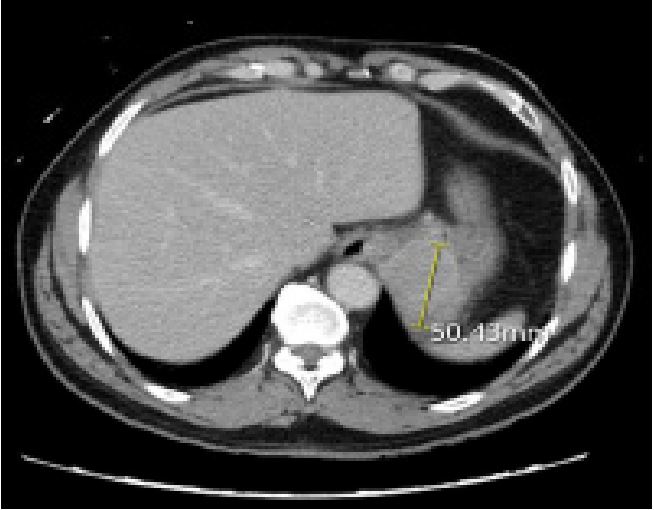
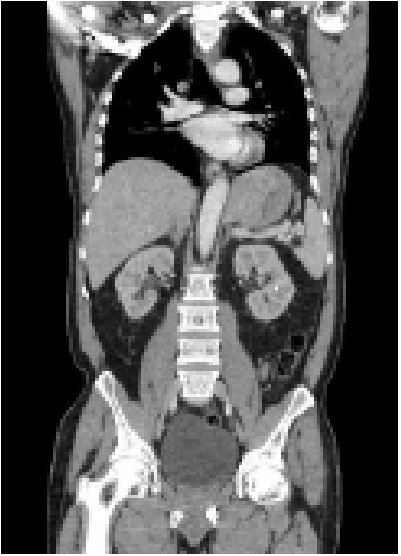
abdomen, and pelvis demonstrated a 5 cm solid mass in the
gastric cardia with distal esophageal involvement (Figure 1). An
EGD was done 4 days after admission that confirmed the presence of a 49.2 x 37.9 mm gastric cardiac mass infiltrating the
gastroesophageal junction and distal portion of the esophagus,
most likely arising from muscularis propria, with celiac lymphadenopathy and multiple malignant lymph nodes. A biopsy of
the lesion came back positive for a poorly differentiated gastric
adenocarcinoma, intestinal type with ulceration, and fragments
of squamous mucosa with distal esophageal involvement. The
patient was discharged with outpatient follow-up but returned
to the ED the following day with an episode of syncope while
eating breakfast. A CT scan of his chest was done on admission
and showed an acute occlusive pulmonary embolism. The use
of anticoagulants was contraindicated due to the risk of heavy
bleeding in our patient; this necessitated the placement of an
inferior vena cava filter. A subcutaneous infusion port was also
placed and the patient underwent 4 cycles of FLOT (Fluorouracil, Leucovorin, Oxaliplatin, and Docetaxel) chemotherapy.
The patient was then referred to surgical oncology planning
for the resection of the gastric adenocarcinoma, which was to
be followed by adjuvant chemotherapy. The plan was made
for a robot-assisted Ivor Lewis esophagogastrectomy. During
the open exploratory laparotomy, it was quickly found that
the stomach adhered to the aorta posteriorly and to the diaphragm. Unable to identify a clear delineation between the aorta and the stomach and safely resect the tumor, the procedure
was abandoned. The patient was discharged 5 days after the
operation, and he is currently undergoing combination chemoradiation, with the possibility of surgery after completion of the
combination therapy.
Discussion
In our patient, the risk of damaging vital structures was too
great to proceed with the removal of the tumor. When the disease process gets to this advanced stage, there are not many
viable options other than palliative chemotherapy. Our patient
presented with alarm symptoms, such as anemia secondary to
bleeding, weight loss, and fatigue, indicating that his disease
The patient does not have a family history of gastric cancer, nor
has he presented to the ED or his PCP with alarm symptoms in the past. The fact that the cancer was diagnosed at a later stage
in this disease process is a compelling reason why the cancer
was able to invade to the extent that it did.
Ideally, early identification and removal via endoscopy
would be the optimal way to treat and eradicate the disease
effectively. Screening and early detection of gastric carcinoma
can extend the lives of affected patients in the future [6]. Discuss a retrospective cohort study from 2008 to 2014 done in
California which demonstrated that ethnicity does play a factor when it comes to the incidence of gastric cancer. Those of
Asian, Hispanic, and black populations have a 50% increased
risk for gastric cancer when compared with the non-Hispanic
white population.
According to Xia et al., patients with early-stage disease who
have resectable tumors with negative margins have a lower burden of circulating tumor DNA (ctDNA) and fewer alterations in
the DNA itself. Compared to commonly tested tumor markers
such as CEA, CA 19-9, ATP, etc, these newer biomarkers offer
more sensitivity and specificity. Long-noncoding RNA and circular RNA levels in serum can also be used to detect the presence
of early gastric cancer and to monitor the extent of invasion
of gastric cancer and if there is a presence of lymphatic metastasis. Incorporating these biomarkers as screening tools for
gastric cancers in at-risk patients could be beneficial to improving the early detection of the disease. Overall, further research
is required to assess the practicality of these markers in cancer
screening but they provide a potential future direction for advancements in early gastric cancer detection [6].
The same report mentioned that under-experienced endoscopists have been known to miss precursor lesions and gastric
cancers. More advanced endoscopic training and Artificial Intelligence (AI) advancements can improve the detection rate of
malignancies. Xia et al. discuss how AI has already been used
to detect gastric polyps, predict Barret’s esophageal metaplasia, and improve endoscopic technology. In China, a novel AI
system called ENDOANGEL-ID has been programmed to learn
retrospective and real-time endoscopic images from over
100,000 patients. It has demonstrated increased sensitivity and
improved early detection of gastric cancer in trials. Another
AI system in Korea called AI-scope has been shown to identify
gastric lesions and measure the depth of invasion. This method
has proved to be superior to endoscopic US. Implementing this
technology can be expensive and requires further resting, but
it can provide a foundation for improved screening of gastric
cancer [6].
The early detection of gastric cancer requires financial and
community support, however, a study by [7] suggested that
endoscopic screening for gastric cancer in high-risk racial and
ethnic groups in the United States at the beginning at age 50
with a surveillance every 3 years is cost-effective. In Japan,
where the incidence of gastric cancer is high, screening with
double-contrast radiographs with photofluorography for gastric
cancer is recommended for patients older than 50 every year.
This practice has been in effect since 1960. The 5-year survival
rate is 15-30% better in those who are screened for the disease
versus those who are diagnosed because of symptom presentation. Just as there is a well-known concrete method for colorectal cancer screening, perhaps a new multi-disciplinary model
for gastric cancer screening is needed, especially for high-risk
populations and ethnic groups [8].
Conclusion
The overall survival rate of those inflicted with advanced gastric adenocarcinoma is low. Gastric cancers are responsible for
783,000 deaths per year, making it the third most deadly cancer
among men in the world [9]. In this report, we discussed the
case of a 58-year-old male patient who had a gastric adenocarcinoma that invaded too far into the posterior aspect of the aorta
to resect safely without damaging the neighboring vital organs.
Given the advanced stage of his disease, there are not many
more options other than a combination of chemotherapy and
radiation with the hope of downstaging the tumor to make it
amenable to surgical resection. We can hope that early screening methods, such as prophylactic endoscopy screening every
few years or testing for more specific biomarkers for gastric cancers, can lead to increased detection, treatment, and survival of
those affected by gastric cancer. By incorporating some of the
methods of screening that countries such as Japan and Korea
use, we can hope to avoid such advanced disease processes as
the one presented in this report. This case had an undesirable
outcome, but it shows us the importance and the need for early
detection and incorporation of screening for gastric cancer in
our medical practice.
References
- Smyth EC, Fassan M, Cunningham D, Allum WH, Okines AFC, et al. Effect of Pathologic Tumor Response and Nodal Status on Survival in the Medical Research Council Adjuvant Gastric Infusional Chemotherapy Trial. Journal of Clinical Oncology. 2016; 34(23): 2721-2727.
- Hu HM, Tsai HJ, Ku HY, Lo SS, Shan YS, et al. Survival outcomes of management in metastatic gastric adenocarcinoma patients. Scientific Reports. 2021; 11(1): 23142.
- Lin D, Khan U, Goetze TO, Reizine N, Goodman KA, et al. Gastroesophageal junction adenocarcinoma: Is there an optimal management? American Society of Clinical Oncology Educational Book. 2019; 39: e88-e95.
- Moureau-Zabotto L, Teissier E, Cowen D, Azria D, Ellis S, et al. Impact of the Siewert Classification on the Outcome of Patients Treated by Preoperative Chemoradiotherapy for a Nonmetastatic Adenocarcinoma of the Oesophagogastric Junction. Gastroenterology Research and Practice. 2015; 404203. https://doi.org/10.1155/2015/404203.
- Huang L, Onaitis M. Minimally invasive and robotic Ivor Lewis esophagectomy. Journal of Thoracic Disease. 2014; 6: S314-S321. https://doi.org/10.3978/j.issn.2072-1439.2014.04.32.
- Xia JY, Aadam AA. Advances in screening and detection of gastric cancer. Journal of Surgical Oncology. 2022; 125(7): 1104-1109. https://doi.org/10.1002/jso.26844.
- Saumoy M, Schneider Y, Shen N, Kahaleh M, Sharaiha RZ, et al. Cost Effectiveness of Gastric Cancer Screening According to Race and Ethnicity. Gastroenterology. 2018; 155(3): 648-660. https://doi.org/10.1053/j.gastro.2018.05.026.
- Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric cancer: Epidemiology, risk factors, classification, genomic characteristics, and treatment strategies. International Journal of Molecular Sciences. 2020; 21(11): 4012. https://doi.org/10.3390/ijms21114012.
- Rawla, Paramvir, Barsouk, Alexander. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Prz Gastroenterol. 2019; 14(1): 26-38. doi: 10.5114/pg.2018.80001.