Introduction
Pancreatic cancer has remained one of the most lethal tumors despite the advancement of medical care, and 80-90% of
which is made up of Pancreatic Ductal Adenocarcinoma (PDAC)
pathologically. Exophytic pancreatic cancer is a rare subtype
of pancreatic cancer. Until now, there is no well-defined notion and derived from morphology instead. That is, the tumor
shows the characteristics of large or all of it located outside
the pancreas and infiltrating outward [1]. At the same time,
the pancreas appears normal without mass. Exophytic pancreatic cancer mostly originates from the uncinate process of the
pancreatic head and a few from the body of the pancreas [2].
Patients with exophytic pancreatic cancer often exhibit hidden
attack and atypical symptoms, resulting in difficulty in identification and diagnosis. Imaging is imperative to diagnosis and
is required to obtain accurate information about anatomy, infiltration, and metastasis. In this paper, we report an unusual
case of exophytic PDAC in a patient with multiple metastases
in β-2-Fluoro-2-deoxy-D-glucose(18F-FDG) Positron Emission Tomography (PET)/Computed Tomography (CT).
Case presentation
A 69-year-old man was admitted to the hospital for reactive
arthritis three months ago. Laboratory tests showed an elevated
carbohydrate antigen 199 (CA199) (1167 U/ml) during his hospitalization. A good performance status was presented during
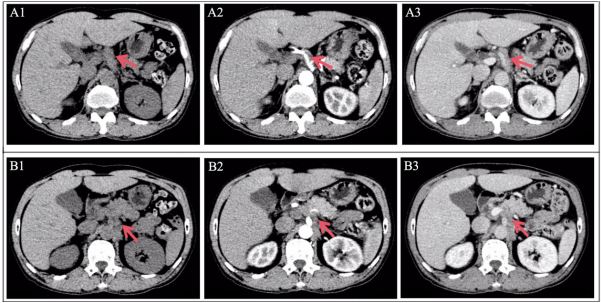
his physical examination. The patient underwent a contrast-enhanced Computed Tomography (CT) of the chest and abdomen
to look for possible causes. Axial images of contrast-enhanced
CT (Figure 1) showed a strip-shaped soft tissue around the coeliac artery with mild enhancement. No noticeable abnormalities were found in other tissues and organs. Subsequently, the
patient was discharged and requested follow-up.
Two months later, the patients began to present with persistent stretching pain throughout the abdomen and a marked decrease in body weight (10 kg). Meanwhile, he had significantly
increased level of CA199 (>12000 U/ml) and other elevated tumor markers, including Carcinoembryonic Antigen (CEA) (7.43
ug/L), Carbohydrate Antigen 125 (CA125) (179.3 U/ml), and
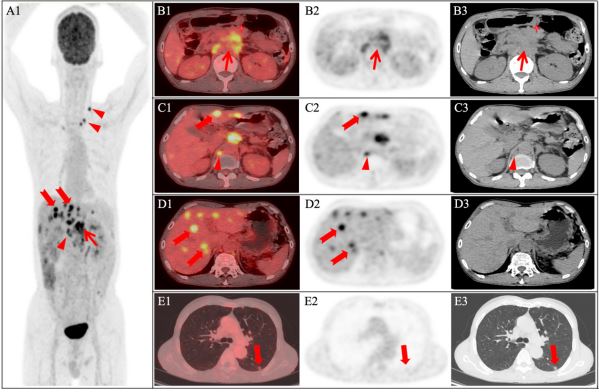
Carbohydrate Antigen 153 (CA153) (37.2 U/ml), strongly suggestive of neoplastic lesions. Therefore, 18F-FDG PET/CT (GE Discovery 710, the United States) was performed for diagnosis and staging. Images revealed that an 18F-FDG-avid soft tissue lump
(Figure 2) in the posterior of the pancreatic body with maximum
standardized uptake value (SUVmax)of 7.7, which was poorly demarcated from the pancreatic body, coeliac artery, and superior
mesenteric artery, and was noticeably larger compared with
two months earlier. In addition, metastatic lymph nodes in the
abdominal cavity, retroperitoneal, and left supraclavicular fossa
with high 18F-FDG uptake (SUVmax 5.9), multiple liver metastases
with high 18F-FDG uptake (SUVmax 9.5), and lung metastases with
mild 18F-FDG uptake (SUVmax 1.5) were detected simultaneously.
Given clinical and imaging findings, 18F-FDG PET/CT suggested a rare diagnosis of exophytic pancreatic cancer with liver, lung,
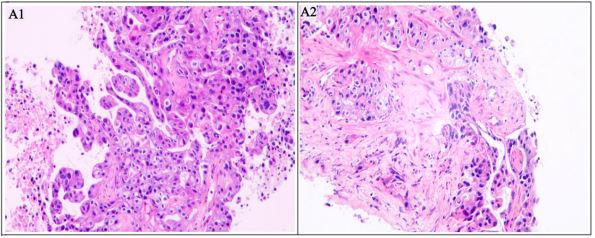
and lymph node metastases. Consequently, the needle biopsy
was performed with the liver lesion, and the liver metastases of
PDAC were pathologically confirmed (Figure 3, low-to-moderate differentiation). The man ultimately received palliative care
with serplulimab (200 mg) immunotherapy because of the advanced stage.
Discussion
Exophytic pancreatic cancer is a little-known entity, which is
characterized by origination derived from the uncinate process
of the pancreatic head mostly and a few from the body of the
pancreas, tumor localization most or all outside the pancreas,
and infiltrating growth. In addition to PDAC, less common pancreatic tumors of exophytic growth include Solid Pseudopapillary Tumor (SPT) and Acinar Cell Carcinoma (ACC) [3]. Unlike
typical PDAC, most exophytic PDAC do not involve the pancreas
and bile ducts. Thus, less jaundice and obstructive pancreatitis
are observed, contributing to a silence onset. It is shown that
abdominal pain and weight loss have been reported in 70%
of tumors occurring in the uncinate process of the pancreatic
head, while jaundice has been reported in less than 28% of patients [4]. Besides, there are no indirect signs such as the typical “two-tube sign”, pancreatic parenchymal atrophy upstream
of the tumor, and retentive cysts/pseudocysts on conventional
imaging. It is worth noting that most patients would experience a markedly elevated CA199 prior to the development of a
significant mass, which may be helpful in suggesting the origin
of the tumor. Locally advanced/advanced PDAC often invades
peripheral nerves and blood vessels of the pancreas, while exophytic PDAC is more pronounced. Importantly, exophytic PDAC
is nourished by peripheral nerves and blood vessels and can
grow and extend along nerve vessels in the early stage [4] (Figure 1), causing abdominal pain when tumor invades adjacent
celiac plexus and diarrhea when it invades the superior mesenteric artery [5].
Microscopically, three components are mainly observed in
PDAC, including tumor cells, tumor stroma and normal pancreatic tissue, and the ratio of them determines the imaging finding of the tumor. Tumors tend to appear as low- or iso-dense
masses on CT and show a lower T1-Weighted Imaging (WI) signal than normal pancreas and a T2WI signal similar to or slightly
higher than that of normal pancreas on Magnetic Resonance
Imaging (MRI). Theoretically, PDAC is a hypovascular tumor
with the features of obvious pro-connective tissue proliferation and interstitial fibrosis. As a result, it is mildly enhanced
in the arterial phase of enhanced scanning (lower than normal
pancreas) and gradually strengthened in the portal or delayed
phase. Due to the special location of the tumor, exophytic PDAC
commonly appears as a retroperitoneal soft tissue mass with
progressive growth, irregular morphology, uniform density and signal. Enhanced CT or MRI discovers the same enhancement
modality as PDAC, which contributes to a clearer boundary to
the pancreas. Moreover, exophytic PDAC is a high-grade malignant entity that often invades the adjacent celiac trunk artery
and superior mesenteric artery (wrapping around the arterial
wall >180°). A previous study showed that the exophytic PDAC
is an FDG-avid tumor with a high uptake of 18F-FDG [6], which
is consistent with our report. In our case, 18F-FDG PET/CT imaging not only identified the primary lesion but also detected
multiple metastases in lymph nodes, liver and lungs, which
provided a significant basis for accurate staging of patients.
Because of atypical clinical symptoms and imaging manifestations, exophytic PDAC is easily misdiagnosed and needs to be
distinguished from diseases such as retroperitoneal lymphoma
and Retroperitoneal Fibrosis (RF). Retroperitoneal lymphoma,
mainly affiliated with non-Hodgkin lymphoma, manifests as enlarged lymph nodes around the aorta preferring fusing into a
clump, moving and surrounding adjacent blood vessels without
invading, which is called an “aortic floatation sign”. After the
injection of contrast medium, the lesion often demonstrates
uniform enhancement, and rare necrosis, cystic changes and
hemorrhage occur. In terms of the 18F-FDG PET, retroperitoneal
lymphoma commonly appears with a significantly high uptake.
RF is characterized by chronic nonspecific inflammation of retroperitoneal tissue with fibrous tissue hyperplasia. Typical imaging findings of RF include retroperitoneal irregular soft-tissue
masses located anterior and lateral to the distal abdominal
aorta or proximal common iliac artery, which can encircle and
compress adjacent vessels without significant vascular displacement. Noticeably, RF is apt to involve the ureters, inferior vena
cava, renal arteries, and mesenteric vessels [7]. There are a variety of enhancement modes observed in RF, which depends on
the maturity of fibrosis. In addition, the uptake degree of the
lesion is correlated with disease course and activity.
Conclusion
In summary, exophytic PDAC tends to present with an insidious onset and atypical clinical symptoms. On laboratory tests,
it is often accompanied by an increased level of CA199. A retroperitoneal mass surrounding the blood vessel and connected
to the pancreas, with the characteristics of infiltrative growth
and lack of blood supply, is the specific and important imaging
sign. Importantly, exophytic PDAC, a high degree of malignancy,
exhibits a high FDG-avid pattern. To our knowledge, this is the
first report showing exophytic PDAC with multiple metastases
detected by 18F-FDG PET/CT. Therefore, our study suggests that 18F-FDG PET/CT is a promising and indispensable tool for diagnosis, differential diagnosis and staging of exophytic PDAC.
Declarations
Ethics and patient consent: Written informed consent has
been obtained from the patient.
Acknowledgments: We acknowledge the Department of
Nuclear Medicine the Second Affiliated Hospital of Guangzhou
Medical University for their excellent care of this patient.
Statement of ethics: The study was conducted according
to the guidelines of the Declaration of Helsinki and approved
by the Ethics Committee of The Second Affiliated Hospital of
Guangzhou Medical University.
Conflict of interest: The authors report no conflicts of interest in this work.
References
- Fang X, Bian Y, Jiang H, Wang L, Shao C, et al. Atypical imaging findings of common pancreatic tumors. Chinese Journal of Digestive Surgery. 2021; 1018-1024.
- Yang TM, Han SC, Wu CJ, Mo LR. Acinar cell carcinomas with exophytic growth and intraductal pancreatic duct invasion: Peculiar multislice computed tomographic picture. J Hepatobiliary Pancreat Surg. 2009; 16: 238-241.
- Adsay NV, Pierson C, Sarkar F, Abrams J, Weaver D, et al. Colloid (mucinous noncystic) carcinoma of the pancreas. The American journal of surgical pathology. 2001; 25(1): 26-42.
- O’Sullivan AW, Heaton N, Rela M. Cancer of the uncinate process of the pancreas: Surgical anatomy and clinicopathological features. Hepatobiliary & pancreatic diseases international: HBPD INT. 2009; 8(6): 569-574.
- Liu C, Tian X, Xie X, Gao H, Zhuang Y, et al. Comparison of uncinate process cancer and non-uncinate process pancreatic head cancer. Journal of Cancer. 2016; 7(10): 1242.
- Sanada Y, Yoshida K, Itoh M, Okita R, Okada M. Invasive ductal carcinoma of the pancreas showing exophytic growth. Hepatobiliary Pancreat Dis Int. 2009; 8(1): 97-102.
- Peisen F, Thaiss WM, Ekert K, Horger M, Amend B, et al. Retroperitoneal fibrosis and its differential diagnoses: the role of radiological imaging. Georg Thieme Verlag KG. 2020; 929-936.