Introduction
Intraosseous hemangiomas or Primary Intraosseous Hemangiomas (PIH) are slow-growing innocuous blood vessel tumors
that make up less than 1% of all bone malignancies [1]. Histologically, hemangiomas are divided into two types: capillary and
cavernous. The cavernous form (cavernomas) accounts for the
majority of cranial hemangiomas, whereas capillary hemangiomas account for the majority of spinal hemangiomas [2,3].
Although Heckl et al. gathered 125 documented cases by the
year 2000, cavernous hemangiomas of the cranial vault remain
uncommon. These lesions are estimated to account for 0.2 percent of all benign skull tumors [4], with a clear female predisposition (3:1) [2].
Calvarial cavernous hemangiomas are cavernous hemangiomas that develop from arteries in the diploic space and are fed
by branches of the external carotid artery. The primary blood
supply of the arteries are the middle meningeal and superficial temporal arteries [5]. Calvarial hemangiomas usually affect
the outer table of the skull and the diploe, with the inner table
remaining relatively unaffected [6]. It’s rare for the inner table
and extradural area to be so involved [7]. Prior trauma to the
region is thought to be the most prevalent cause of intraosseous hemangiomas. They bleed profusely when removed or biopsied, therefore preoperative diagnosis of the lesion’s vascular
character is critical [8]. There are four different variants of hemangiomas: Capillary type, Cavernous type, Mixed variant, and
Scirrhous type [8]. They are most frequently seen in the vertebral skeleton, although they can also be found in the calvarium
and facial bones. The temporal bone is the most prevalent place
in the head, followed by the parietal, mandible, malar, and zygomatic areas [8].
In this case report we discuss the case of 9 years old male
who presented with a large soft mass in its right parietal region.
Case report
A 9-year-old boy was brought into OPD by his parents after
they noticed a scalp swelling 2 months back. The swelling has
progressively increased in size in the last 2 months. There was
no history of trauma to the scalp, vertigo, vomiting, headaches,
or fits.
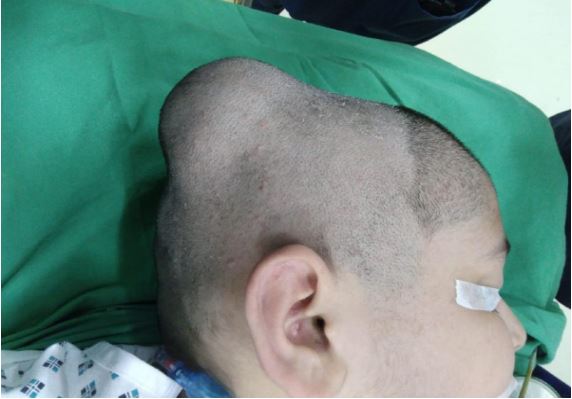
On examination, there was an active, alert child with a right
parietal swelling. It was 6x6 cm in dimension, present in the
right parietal region, firm in consistency, and the overlying skin
normal with no marks, punctum, or striae. Skin was adherent to
swelling, and swelling was adherent to bone. His neurological
examination was unremarkable.
An ultrasound examination of the scalp revealed a large,
well-defined, heterogeneous soft tissue mass lesion in the right
parietal region with an underlying bone osteolytic appearance,
a hyperechoic sclerotic rim, and thickened bone trabeculae
with internal hypervascularity in Doppler examination.
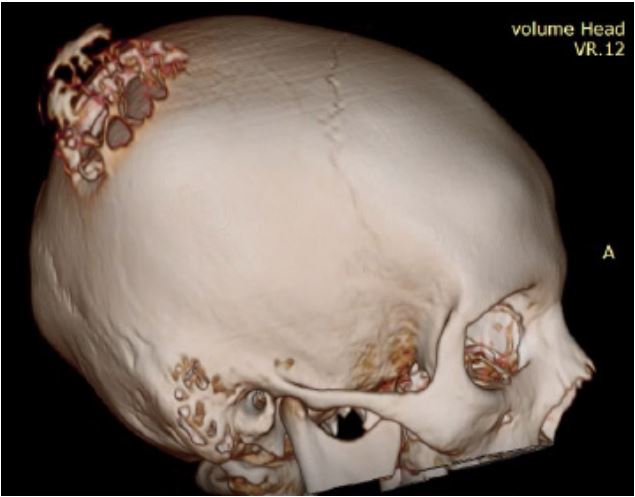
A CT scan of the brain with contrast was advised, which
showed a large lytic expansile skull bone lesion at the right parietal bone, approximately 55x50x48 mm in AP, transverse, and
CC dimensions, respectively. It showed a well-marginated outline with an incomplete sclerotic rim with erosions of both internal and external tables and a radiating trabecular thickening
(spoke wheel) appearance. Dense enhancement was observed
following IV contrast vascular. It showed smooth extracranial
and intracranial bulges with localized mass effects seen as effacement to the right parietal lobe, but no midline shift was
seen. The findings likely represented an aneurysmal bone cyst.
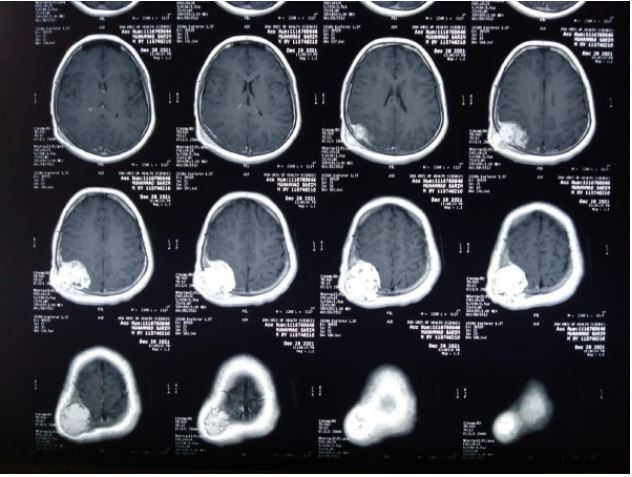
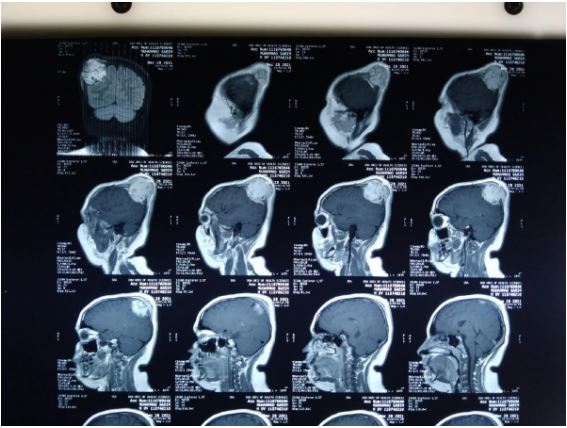
An MRI examination was also done and was consistent with
CT findings, but no fluid levels were seen, making the diagnosis
of an aneurismal bone cyst less likely.
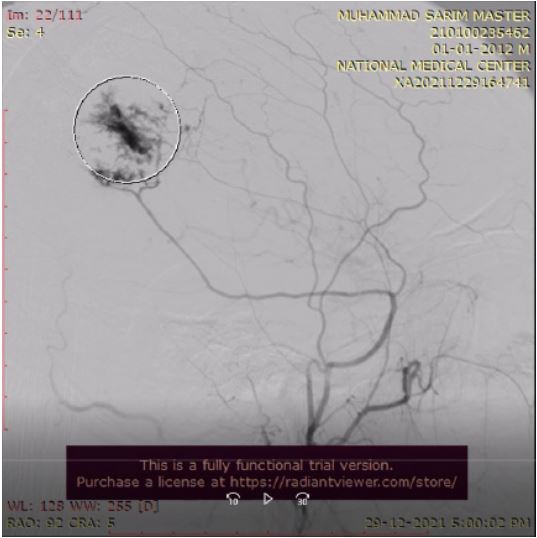
An angiogram of the lesion was also done, showing filling by
the posterior division of the right superficial temporal artery.
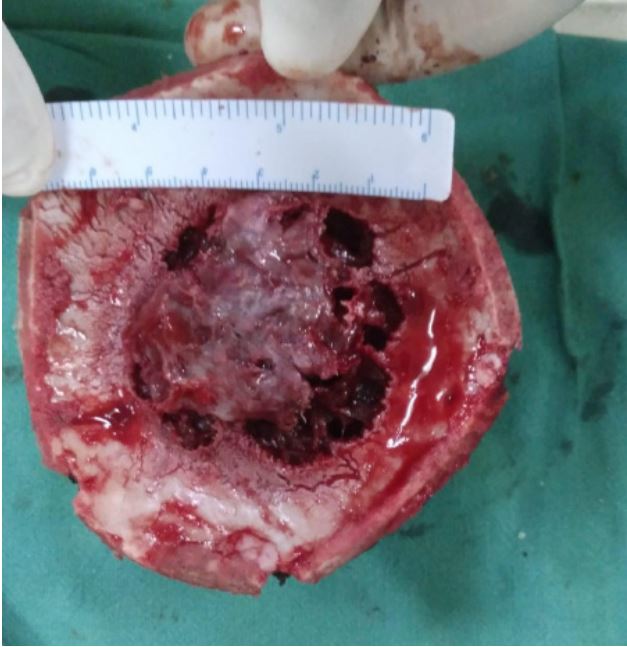
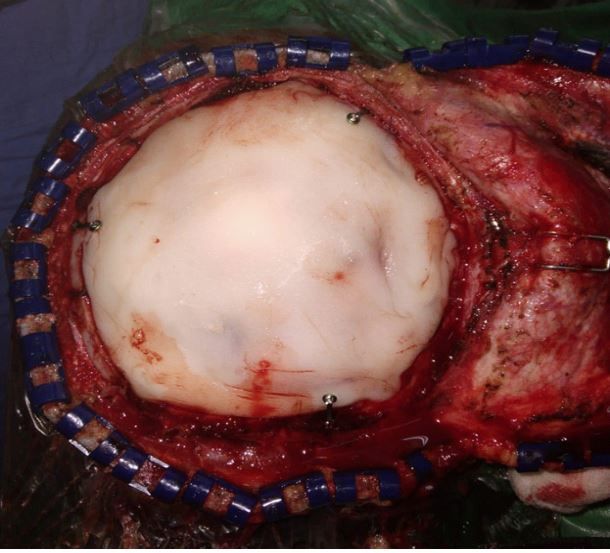
The patient was prepared for surgery after having given informed consent. An incision was made around the swelling, a
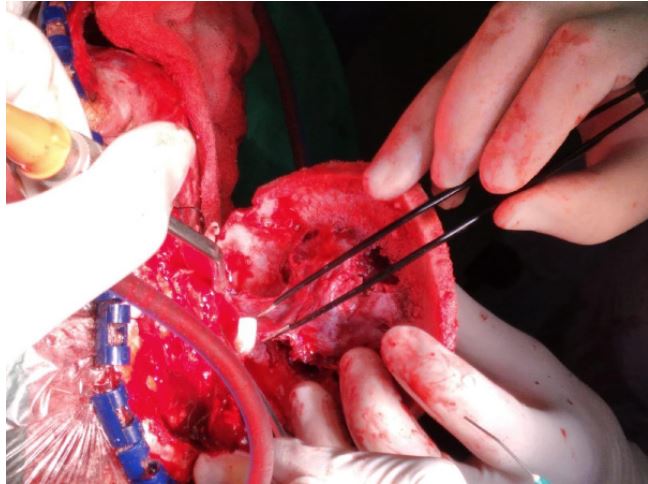
flap was raised, and a craniotomy was performed around the
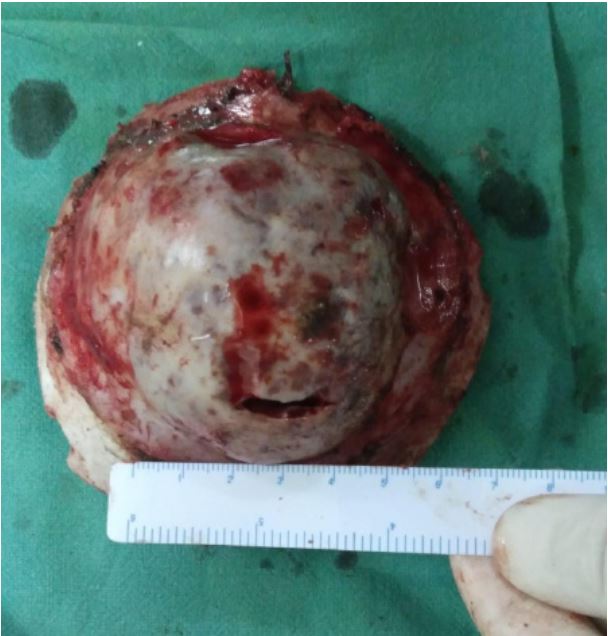
lesion. The bone flap was carefully separated from the dura,
which was intact. Hemostasis was secured and layered closure
was done.
The post-operative stay of the patient was uneventful. A biopsy showed an intraosseous cavernous hemangioma.
Discussion
Vasoformative tumors encompass a wide range of benign
and malignant neoplasms. Hemangiomas are benign localized
tumors that arise from the endothelium of blood vessels [9].
These are further classified based on the size of the proliferating vascular areas, which range from minor to major: capillary, cavernous, and cellular. Large, dilated, blood-filled cavities
with a flattened endothelium lining are the pathologic look of
cavernous hemangiomas [9]. It’s crucial to rule out malignant
vasoformative tumors like angiosarcoma or spindle cell hemangioma by obtaining a pathologic diagnosis of these diseases [9].
Toynbee documented a vascular tumor growing inside the
boundaries of the parietal bone in 1845, which was the first
description in the English literature [10]. The temporal bone
is assumed to be the most typically afflicted, followed by the
parietal bone and the temporal and occipital bones, which are
affected less frequently [11]. They are generally solitary [2]. Intraosseous cavernous hemangiomas are slow-growing lesions
that most commonly affect women in their second to fourth decades [6,12,13]. Only 9% of cases are discovered in the first ten
years [14]. There is also one rare presentation of a Cavernoma
along with hemangioma in a 72 years old lady [15]. Pain and a
visible or palpable bony hard mass that is slowly expanding and
covered by normal skin are the most common symptoms [12].
Because these tumors tend to develop externally, neurologic
impairments are uncommon, but intracranial enlargement has
also been described in rare cases [16].
The signal characteristics of vertebral PIHs differ significantly
from those of skull PIHs on radiography. On cross-sectional computed tomography scans, in thin cuts of bone window, vertebral
hemangiomas appear as well-circumscribed expansile regions
with bone remodeling in the form of a pathognomic “polka dot”
pattern [17]. Hemangiomas of the skull do not have this distinctive pattern. The quantity of venous flow and lipid content within a lesion considerably influences MRI characteristics, resulting
in mottled heterogeneous signal seen on T1- and T2-weighted
sequences [17,18]. The delayed contrast blush is seen in larger
PIHs of the skull, but not in smaller lesions, especially those affecting the base of the skull [18].
On radiologic examination, a calvarial cavernous hemangioma’s classic sunburst appearance is attributable to osteoblastic
remodeling with trabecular bone after tumor osteoclastic activity [16,19]. Preoperative diagnosis is challenging, and histology is required for a definite diagnosis [1,11]. The cavernous
variety accounts for the majority of calvarial hemangiomas [20].
The cavernous type is made up of a cluster of massive, dilated
blood arteries separated by fibrous tissue, which matched our
observations. Vertebral hemangiomas, on the other hand, are
most commonly capillary in nature, with no fibrous septa and
a smaller vascular lumen [21]. Mixed-type hemangiomas comprise components of both cavernous and capillary forms [16].
Capillary hemangiomas have the potential to develop into cavernous hemangiomas [22].
PIHs do not naturally retreat, necessitating a decisive therapy. Surgery, radiation, and embolization prior to surgery are
all possibilities for treating cranial hemangiomas [23]. Because
imaging results are not specific, total excision of the lesion is the
treatment with the best prognosis [11]. Histopathologic diagnosis is also required because imaging findings are not specific
[11]. The risk of bleeding is reduced when total resection is performed with an acceptable normal bone margin [2,11,23,24].
Preoperative embolization has also been observed to minimize
intraoperative blood loss by several researchers [19,25,26]. Radiotherapy can slow the development of tumors but cannot remove them [11].
References
- Vural M, Acikalin MF, Adapinar B, Atasoy MA. Congenital cavernous hemangioma of the calvaria. Case report. Journal of neurosurgery Pediatrics. 2009; 3(1): 41-5.
- Heckl S, Aschoff A, Kunze S. Cavernomas of the skull: Review of the literature 1975-2000. Neurosurgical review. 2002; 25(1-2): 56-67.
- Nasrallah IM, Hayek R, Duhaime AC, Stotland MA, Mamourian AC. Cavernous hemangioma of the skull: surgical treatment without craniectomy. Journal of neurosurgery Pediatrics. 2009; 4(6): 575-9.
- Yoshida D, Sugisaki Y, Shimura T, Teramoto A. Cavernous hemangioma of the skull in a neonate. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1999; 15(6-7): 351-3.
- Verma SK, Singh PK, Garg K, Satyarthee GD, Sharma MC, et al. Giant calvarial cavernous hemangioma. Journal of pediatric neurosciences. 2015; 10(1): 41-4.
- Naama O, Gazzaz M, Akhaddar A, Belhachmi A, Asri A, et al. Cavernous hemangioma of the skull: 3 case reports. Surgical neurology. 2008; 70(6): 654-9.
- Xu P, Lan S, Liang Y, Xiao Q. Multiple cavernous hemangiomas of the skull with dural tail sign: A case report and literature review. BMC neurology. 2013; 13: 155.
- Powers DB, Fisher E, Erdmann D. Zygomatic Intraosseous Hemangioma: Case Report and Literature Review. Craniomaxillofacial trauma & reconstruction. 2017; 10(1): 1-10.
- Bolous Y, Bullock M, Clarke DB, Massoud E. Intraosseous Cavernous Hemangioma of the Middle Turbinate: A Case Report. Ear, nose, & throat journal. 2021: 145561320984581.
- Toynbee J. An account of two vascular tumors developed in the substance of bone. Lancet. 1845; 2: 676.
- Yang Y, Guan J, Ma W, Li Y, Xing B, et al. Primary Intraosseous Cavernous Hemangioma in the Skull. Medicine (Baltimore). 2016; 95(11): e3069-e.
- Ajja A, Oukacha N, Gazzaz M, Akhaddar A, Elmostarchid B, et al. Cavernous hemangioma of the parietal bone. A case report. Journal of neurosurgical sciences. 2005; 49(4): 159-62.
- Vega A, De Obieta E, Aguado G, Esqueda M, Ruíz S, et al. Multifocal cavernous hemangioma of the skull. Case report. Neurocirugia (Asturias, Spain). 2010; 21(6): 484-90.
- Martínez-Lage JF, Torroba MA, Cuartero Pérez B, Almagro MJ, López López-Guerrero A, et al. Cavernous hemangiomas of the cranial vault in infants: a case-based update. Child’s nervous system: ChNS: Official journal of the International Society for Pediatric Neurosurgery. 2010; 26(7): 861-5.
- Kilani M, Darmoul M, Hammedi F, Ben Nsir A, Hattab MN. Cavernous hemangioma of the skull and meningioma: association or coincidence? Case reports in neurological medicine. 2015; 2015: 716837.
- Liu JK, Burger PC, Harnsberger HR, Couldwell WT. Primary Intraosseous Skull Base Cavernous Hemangioma: Case Report. Skull base: official journal of North American Skull Base Society [et al]. 2003; 13(4): 219-28.
- Singh U, Kalavakonda C, Venkitachalam S, Patil S, Chinnusamy R. Intraosseous Hemangioma of Sella: Case Report and Review of Literature. World Neurosurg X. 2019; 3: 100030.
- Noblett DA, Chang J, Toussi A, Dublin A, Shahlaie K. Hemangioma of the Cavernous Sinus: A Case Series. Journal of neurological surgery reports. 2018; 79(2): e26-e30.
- Salunke P, Sinha R, Khandelwal NK, Kumar A, Gupta K, et al. Primary intraosseus cavernous hemangioma of the skull base. British journal of neurosurgery. 2010; 24(1): 84-5.
- Khanam H, Lipper MH, Wolff CL, Lopes MB. Calvarial hemangiomas: Report of two cases and review of the literature. Surgical neurology. 2001; 55(1): 63-7.
- Nasi D, Somma L, Iacoangeli M, Liverotti V, Zizzi A, et al. Calvarial bone cavernous hemangioma with intradural invasion: An unusual aggressive course-Case report and literature review. International journal of surgery case reports. 2016; 22: 79-82.
- Tsao MN, Schwartz ML, Bernstein M, Halliday WC, Lightstone AW, et al. Capillary hemangioma of the cavernous sinus. Report of two cases. Journal of neurosurgery. 2003; 98(1): 169-74.
- Prasad GL, Pai K. Pediatric cranial intraosseous hemangiomas: A review. Neurosurgical review. 2018; 41(1): 109-17.
- Cervoni L, Artico M, Delfini R. Intraosseous cavernous hemangioma of the skull. Neurosurgical review. 1995; 18(1): 61-4.
- Banerji D, Inao S, Sugita K, Kaur A, Chhabra DK. Primary intraosseous orbital hemangioma: a case report and review of the literature. Neurosurgery. 1994; 35(6): 1131-4.
- Muzumdar D, Goel A, Desai K, Bhayani R, Sharma P. Primary hemangioma of the occipital bone in the region of the torcula--two case reports. Neurologia medico-chirurgica. 2002; 42(1): 27-30.