Introduction
Small Bowel Obstruction (SBO) is a frequently encountered
emergency surgical presentation. A large number of the cases
are caused by postsurgical adhesions and hernias.
Gastrointestinal Tract (GIT) lymphomas comprise approximately 1-4% of all Gastrointestinal (GI) malignancies, 10-15 %
of all Non-Hodgkin’s Lymphoma (NHL) and 30-40% of all extra nodal NHL’s, making the GIT the most frequent site of extra nodal lymphomas [1]. Common sites of NHL are stomach,
small bowel and ileocaecal region (stomach being the most
common). B-cell type is found to be common in majority of the
cases of primary intestinal NHL with the most common subtype
being Diffuse Large B Cell Lymphoma (DLBC).
A large number of cases of primary intestinal NHL are B-Cell
type with DLBC being the most common subtype.
Lack of specific symptoms causes a delayed diagnosis which
is usually made postoperatively. Diagnosis can be made based
on endoscopic biopsies and a histopathology report, subtyping
can be done using immunological and molecular markers. In
view of a rare disease phenomenon a proper treatment protocol is lacking. The principle modality of treatment is combined;
surgery and chemotherapy.
In this report, the authors present a rare case of small bowel
obstruction which was clinically diagnosed as obstruction due
to post operative adhesions but was, in fact, DLBCL.
Case presentation
A 30 year old, non-diabetic, normotensive female patient
presented to the surgery emergency with complaints of abdominal pain and bilious vomiting for the last 1 week. It was associated with abdominal distension and constipation. There was no
other significant history except having been operated by a pfannenstiel incision for a C-section 6 months ago. She developed a
lump at the lateral edge of the incision, was diagnosed with scar
endometriosis and underwent excision of the scar.
Clinical examination revealed a pulse rate of 98 beats per
minute and BP was 110/70 mm Hg. There was no pallor, icterus,
clubbing, koilonychia or lymphadenopathy. On per abdominal
examination central abdominal distension was present with a
vague palpable mass in the right iliac fossa. X- Ray examination
showed multiple air fluid levels and hence a clinical diagnosis of small bowel obstruction likely due to post surgical adhesions
was made. Since vital signs of the patient were stable, she was
kept on conservative management (NPO, nasogastric aspiration
and IV fluids).
In view of failure of conservative management (vomiting on
oral trial and increase in the size of the lump), patient was taken
up for emergency exploratory laparotomy with a working diagnosis of small bowel obstruction due to post surgical adhesions.
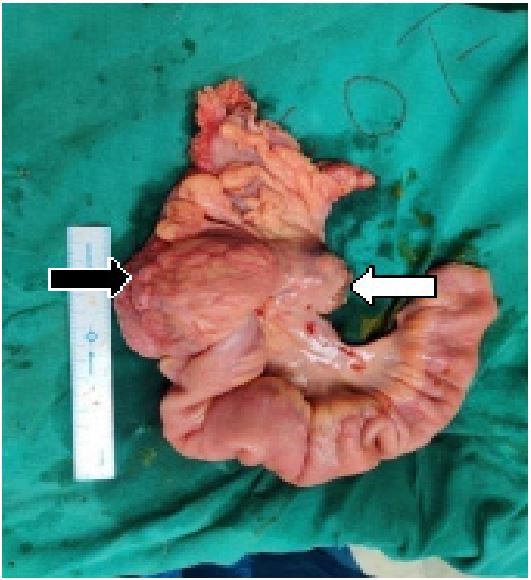
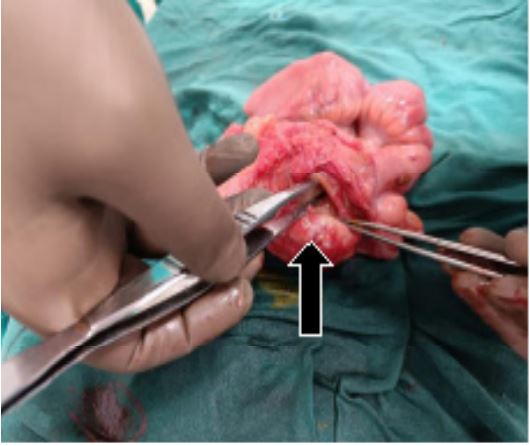
Intraoperatively the following findings were noted (Figure 1
and 2).
1. Level of obstruction was identified at the ileocaecal junction with gross dilatation of the proximal small bowel and
collapsed ascending, transverse, descending colon.
2. A hard intraluminal lump of the size 7x6 cm was present
in the caecum in posteromedial aspect compressing upon
the ileocaecal junction.
3. Mesenteric lymphadenopathy was also present in the
mesentery of right colon and terminal ileum.
Right hemicolectomy with end to end ileotransverse anastamosis was done. Patient was allowed orally on the third post
operative day and was discharged on the fifth post operative
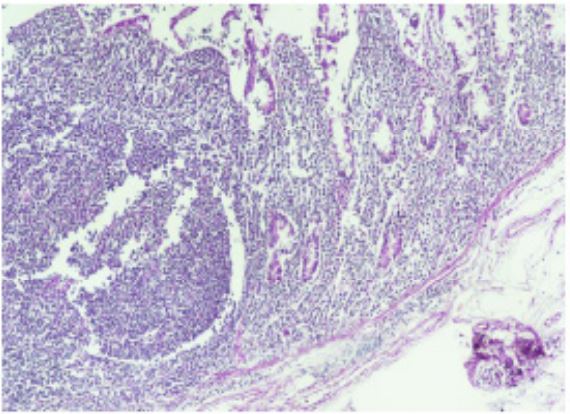
day. Biopsy report of the hemicolectomy specimen confirmed
the diagnosis of NHL, DLBC type arising from the caecum with
tumour nodules in the associated mesenteric lymph nodes.
After receiving the biopsy report, bone marrow biopsy was
done which was normal. With a diagnosis of primary colorectal
lymphoma the patient was started on adjuvant chemotherapy
in the follow up period by a medical oncologist.
Discussion
SBO is a frequently encountered emergency surgical presentation. The vast majority of cases are caused by post-surgical
adhesions and hernias. Malignancy is responsible for approximately twenty percent of cases and is the third most common
cause of SBO [2]. Both benign and malignant tumours can cause
obstruction and they may be found within or outside the small
bowel wall.
Lymphomas make up an estimated 24% of neoplasm induced bowel obstruction[3]. GI lymphomas (an uncommon disease) are the most frequent site of extra nodal lymphomas and
are mostly of NHL type. GI lymphomas comprise approximately
1-4% of all GI malignancies, 10-15% of all NHL and 30-40% of
all extra nodal NHL’s, making the GIT the most frequent site of
extra nodal lymphomas[1]. Order of involvement is as follows;
stomach (65%), small intestine (20-30%), colon (10-20%) and
esophagus (1%)[4].
Most common sites in the colon is the ileocaecal region followed by caecum and sigmoid colon. In their case series, Bairey
et al. had found the ileocaecal region to be the most frequent
site accounting for 76% of cases[5]. As for our case, the area
involved was also the ileocaecal region.
Most of the cases of primary intestinal NHL are B-Cell type
with DLBC being the most common subtype. T cell lymphomas
account for a lesser percentage, however B cell lymphomas
have a better prognosis. Guo-Bao Wang et al. in their case series
of 81 patients found that 51.9% patients had high-grade B-cell
lymphoma and 25.9% patients had T-cell lymphoma [6].
Dawson et al. in 1961 has given the criteria for diagnosis of
primary colorectal lymphomas as follows[7];
1. Absence of peripheral lymphadenopathy at the time of
presentation.
2. Lack of enlarged mediastinal lymph nodes.
3. Normal total and differential white blood cell count.
4. Predominance of bowel lesion at the time of laparotomy
with only lymph nodes obviously affected in the immediate vicinity.
5. No lymphomatous involvement of liver and spleen.
All of the above mentioned criteria were fulfilled by our patient.
The clinical presentation is variable with non-specific abdominal complaints. The most reported symptoms are pain in (62%)
of the patients, abdominal mass (54%) and weight loss (43%)
[8]. It is due to these nonspecific symptoms in more than half of
the patients leading to a delayed diagnosis causing lymphoma
to be a bulky disease reaching up to 5 cm in diameter[5]. Such
large lesions are prone to producing obstruction, bleeding and
perforation like in our case. Due to delay in diagnosis operative procedure is either urgent or emergent for indications such
as obstruction and bleeding. In these case scenarios mostly the
diagnosis is made after operative intervention after the final biopsy report is received. Colonoscopy is an important diagnostic
tool in the armamentarium but is not always possible to diagnose due to lack of specific symptoms in the form of bleeding
per rectum[5].
Most lymphomas can be seen on CT scan as a mass or thickening of the intestinal wall, in rare cases luminal obstruction
may be observed [9] (as present in this case). Diagnosis can be
made based on endoscopic biopsies and a histopathology report, subtyping can be done using immunological and molecular markers.
In view of a rare disease phenomenon a proper treatment
protocol is lacking. The principle modality of treatment is
combined; surgery and chemotherapy. Early stage tumors are
treated with surgery followed by chemotherapy and advanced
stage tumors are treated with multiple modality chemotherapy.
Using Rituximab, B cell lymphomas may be treated with a combination of chemotherapy and immunotherapy for a better response [10].
Conclusion
Primary colonic lymphoma presenting as an intestinal obstruction is a very rare condition. Although it commonly occurs
in an older age group (over 50) it can occur atypically in younger
patients as in this case. The presentation is non-specific leading
to diagnosis at a late stage.
Lymphoma should be kept in mind in diagnosis (using Dawson et al. criteria) when any other cause of obstruction is not
evident. Treatment includes surgical resection of lymphoma followed by chemotherapy.
References
- F d’Amore H, Brincker K, Gronbaek, et al. Non-Hodgkin’s lymphoma of the gastrointestinal tract: A population-based analysis of incidence, geographic distribution, clinicopathologic presentation features, and prognosis Danish Lymphoma Study Group, J Clin Oncol. 1994; 12: 1673-1684.
- CI Ripamonti, AM Easson, H Gerdes. Management of malignant bowel obstruction, Eur J Cancer. 2008; 44(8): 1105-1115.
- MA Beltran, KS Cruces. Primary tumors of jejunum and ileum as a cause of intestinal obstruction: A case control study, Int J Surg. 2007; 5(3): 183-191.
- A Psyrri, S Papageorgiou, Economopoulos T. Primary extranodal lymphomas of stomach: Clinical presentation, diagnostic pitfalls and management, Ann Oncol. 2008; 19(12): 1992 -1999.
- O Bairey, R Ruchlemer, O Shpilberg. Non-Hodgkin’s lymphomas of the colon, Isr Med Assoc J. 2006; 8: 832-835.
- G Wang, G Xu, et al. Primary intestinal non-Hodgkin’s lymphoma: A clinicopathologic analysis of 81 patients, World J Gastroenterol. 2011; 17(41): 4625-4631.
- IM Dawson, JS Cornes, BC Morson. Primary malignant lymphoid tumors of the intestinal tract. Report of 37 cases with a study of factors influencing prognosis, Br J Surg. 1961; 49: 80-89.
- CW Fan, CR Changchien, JY Wang, et al. Primary colorectal lymphoma, Dis Colon Rectum. 2000; 43: 1277-82.
- D Moraitis, P Singh, R Jayadevan, CG Cayten. Colonic wall thickening on computed tomography scan and clinical correlation. Does it suggest the presence of an underlying neoplasia? Am Surg. 2006; 72: 269-271.
- MS Czuczman, AJ Grillo-Lopez, CA White, et al, Treatment of patients with low-grade B-cell lymphoma with the combination of chimeric anti-CD20 monoclonal antibody and CHOP chemotherapy, J Clin Oncol. 1999; 17:268-276.