Introduction
Anaplastic Thyroid Carcinoma (ATC) is known as a highly aggressive type of cancer with a very poor prognosis [1,4]. ATC is a
form of undifferentiated thyroid carcinoma and represents 1-2%
of all thyroid tumours [2,5,6]. Despite being extremally rare, it
accounts for up to 50% of all thyroid cancer-related mortalities
[2,3]. The median survival rate varies, usually 3 to 6 months
after the diagnosis [1,2,4,7,8]. The overall 1-year and 5-year
survival is 10-20% and less than 10% respectively [4,6,7]. ATC’s
origin is unknown. As a form of undifferentiated thyroid cancer
it can arise de novo or from a pre-existing well-differentiated
thyroid tumour due to the accumulation of genetic alterations
[6,9,14]. ATC is exceptional because of its high aggressiveness,
fast growth, and strong invasiveness [5,6]. This type of cancer often presents with metastases to local and distant lymph
nodes, lungs, and liver [15,16]. ATC is more common in women
and people over the age 60 [2,8,15,17,18]. Distant metastases, age, and socioeconomic status are other known risk factors associated with poorer survival in these patients [15,19]. Various
strategies of treatment are used for ATC such as surgical resection, radiation therapy, chemotherapy, immunotherapy, or a
combination of different treatment methods [8,20]. Usually, the
treatment of choice depends on the specific situation [20,21].
Little is known about extrathyroidal ATC. We represent a current knowledge about ATC in extrathyroidal area focusing on
the primary retroperitoneal tumours. We also describe a very
unique case of a patient with a huge primary retroperitoneal
tumour of ATC origin with normal function and structure of thyroid gland.
Case report
A 72-year-old woman was admitted to our hospital due to a
high fever of up to 42 degrees Celsius, acute abdominal pain,
and loss of consciousness. Increasing abdominal growth and
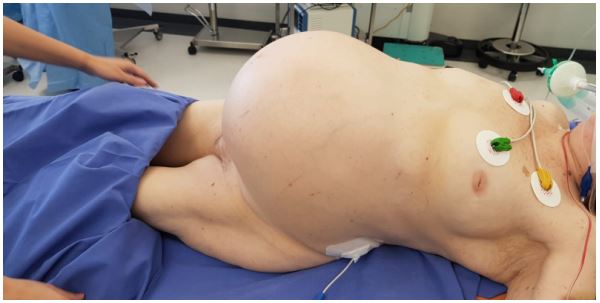
abdominal pain lasted for more than a week. Abdomen ultrasound showed a massive cystic-solid abdominal mass causing
mass effect on surrounding organs.
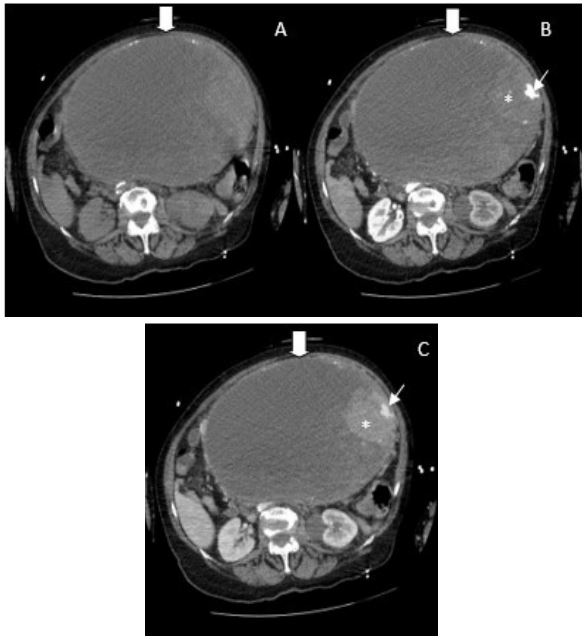
Computed Tomography (CT) imaging was made for diagnostic clarification, huge. ~26x23x27.5 cm in homogeneous (cysticsolid mass with calcinates) abdominal mass. (likely to be an
ovarian tumour) was detected causing left hydronephrosis (Figure 1) (Large intraabdominal well defined mass (large arrow)
with periferal calcifications, predominant cystic component and
solid contrast enchancing tissue (thin arrow) by the left wall,
where a small arterial pseudoaneurysm (asterix) is seen), 2).
Illness anamnesis, health, and family history were unknown
at that time due to severe patient status. Later the patient explained that she noticed an increasing size of her abdomen over
many years (around 30 years) but she did not consult with the
doctors. During check-up in the emergency department patient
was conscious but assessed as having SCORE 12 on Glasgow
Coma Scale (GCS), hemodynamically unstable, hypotonic and
having respiratory failure (hypoxia, acidosis). Increased inflammatory indicators (C-reactive protein 167 mg/l), procalcitonin
(174 µg/l), D-dimmers (31670 µg/L), lactate (>5 mmol/l), anaemia (haemoglobin 109 g/l) and coagulation indicators imbalance were detected. Due to large abdominal mass compression
of the left ureter which caused left hydronephrosis urologist
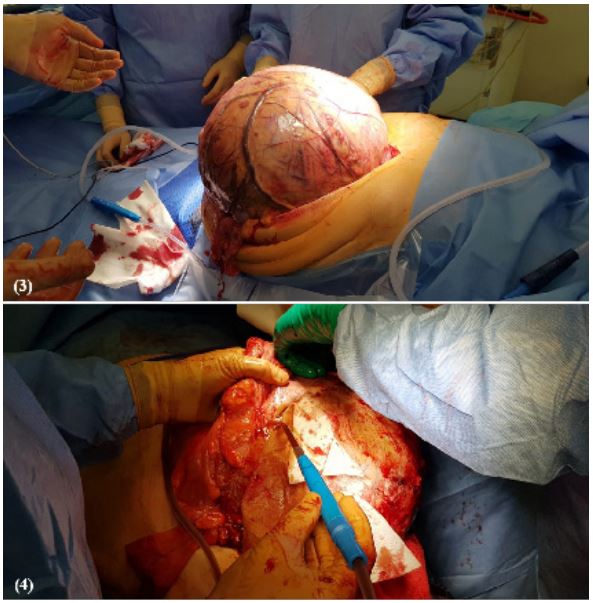
performed a left percutaneous nephrostomy. The patient underwent an urgent operation: extirpation of retroperitoneum
tumour with left hemicolectomy and formation of an end colostomy. The abdominal cavity was occupied with pathological
retroperitoneal mass in the mesentery of large intestines with a
very close connection with descending and sigmoid colons and
also invaded arteria mesenterica inferior (Figure 4). The postoperative course was smooth. After the operation conservative
treatment was provided: antibiotic therapy, vasopressors, analgesics, infusion therapy, anticoalition prevention, and other. In
dynamics, the patient’s condition improved, inflammatory and
uremia indicators decreased, and left kidney nephrostomy was
removed. The patient was discharged, and the whole duration
of hospitalization was less than two weeks. During the late postoperative period the patient had no health complaints. At that
time, results of histopathological analysis of the retroperitoneal
tumour came out: thyroid papillary carcinoma with anaplastic
carcinoma differentiation, invasion of anaplastic carcinoma to
the large intestine. After a month the patient was consulted
repeatedly. Blood tests (thyrotropin-releasing hormone, antithyroglobulin, thyroglobulin, a carcinoembryonic antigen) were
done, and results were in a normal range. A thyroid ultrasound was done, and only minor insignificant diffuse changes were
seen in thyroid tissue and one supraclavicular pathological
lymph node (7x15 mm) was detected on the left. Neck, chest,
abdomen, and pelvic CT was performed two months after the
operation, borderline left subclavicular lymph node (14x10 mm)
was seen, and no focal metastases in the lungs and internal organs were detected. The future treatment plan was discussed
by the multidisciplinary group of doctors (abdominal surgeon,
oncologist-chemotherapist, radiologist, endocrinologist). The
patient was suggested to undergo a positron emission tomography (PET) scan, and later have a biopsy taken from the borderline left subclavicular lymph node, no adjuvant treatment was
suggested at that time. However, the patient refused further
investigation and treatment. Overall, the patient survived more
than 5 months after the operation, no adjuvant treatment was
given. The patient did not have any complains, and no signs of
disease recurrence or metastases were seen except for the borderline left subclavicular lymph node. In addition, the patient is
still alive 2 years after the primary operation and initial diagnosis of primary retroperitoneal cancer with ATC differentiation.
A detailed description of histopathological analysis was done.
The resected retroperitoneal tumour is a thyroid papillary carcinoma with anaplastic carcinoma differentiation and invasion
to the large intestine. The resected yellow-grey retroperitoneal
mass measuring 30 cm in the greatest dimension with necrotic areas and haemorrhages in the centre. Extensive sampling
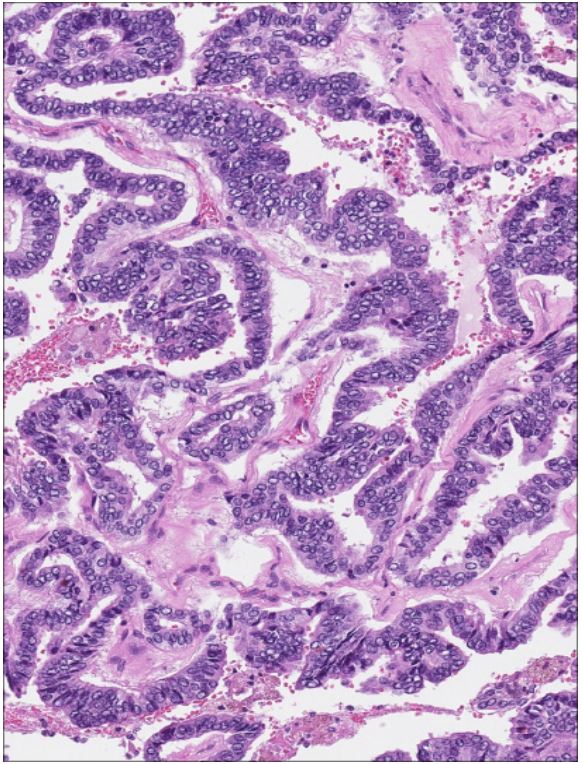
showed various sizes of follicular, glandular and papillary structures, atypical in shape epithelium cells with eosinophilic cytoplasm and oval, round slightly polymorphic nuclei. Little mitosis
was found. The malignant tumour was encapsulated. Less than
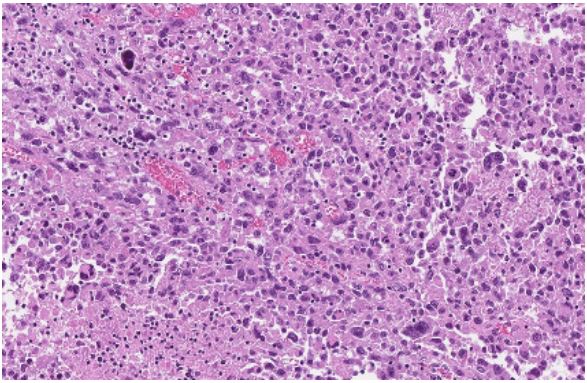
5% of the tumour was composed of large atypical epithelioid
cells with eosinophilic cytoplasm, polymorphic, vesicular nuclei,
and conspicuous nucleoli and high mitotic activity (anaplastic
transformation). More clusters of atypical cells were found in
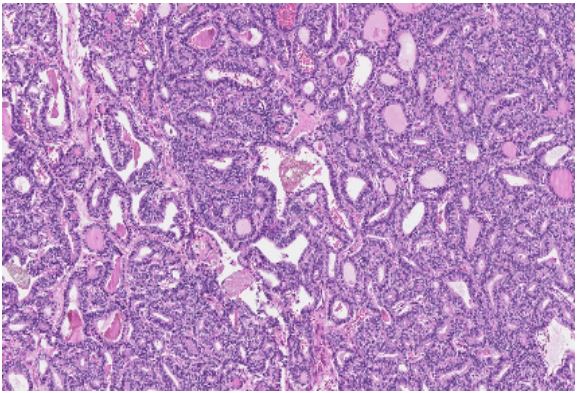
fibrotic capsules with overgrowth (Figure 5-7 (High-grade tumour component, making up 5% of total tumour area, is composed of large epithelioid cells with eosinophilic cytoplasm,
polymorphic nuclei with extensive necrosis, apoptotic and mitotic figures. Some tumour cells are large with multiple nuclei
(Photomicrograph, HE stain))). Immunohistochemical staining
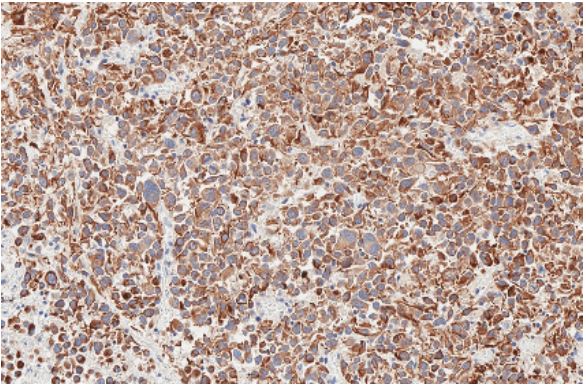
was also performed. The tumour cells were strongly positive
for Anti-Cytokeratin (Cam5.2), Pan-Cytokeratin (PanCK), Cytokeratin 19 (CK19), thyroglobulin, and Thyroid transcription factor-1 (TTF-1). Half of the cells showed positive results for PAX8
and Epithelial membrane antigen (EMA), and less than 5% of
cells – protein Ki67. P53, BRAF, WT1, synaptophysin, estrogen
receptors were negative (Figure 8). Histological analysis and the
overall immunohistochemical profile were concluded to final
diagnosis of a primary retroperitoneal tumour of thyroid papillary carcinoma with focal transformation to thyroid anaplastic
carcinoma of high grade (G4) malignancy (anaplastic carcinoma
giant cell carcinoma type).
Anaplastic carcinoma can be found in the retroperitoneal
area due to several reasons: as primary thyroid cancer, as a
struma ovarii with malignant transformation and metastases to
the retroperitoneal area, or as a metastases of papillary cancer
with anaplastic transformation when a primary tumor is found
in the thyroid. According to the blood tests and imaging results,
the patient in our case presented with primary retroperitoneal
cancer with differentiation of ATC. Written informed consent
from the patient for the publication of any possible identifiable
information (images and case details) was obtained.
Discussion
Anaplastic thyroid carcinoma is known as a very rare and
lethal thyroid cancer [1,3,6]. Little is known about ATC in the
extrathyroidal area. The origin of retroperitoneal cancer with
anaplastic transformation can be explained as a primary thyroid
cancer with anaplastic transformation, as a struma ovarii with
malignant transformation and metastases to the retroperitoneal area, as a metastases of papillary cancer with anaplastic
transformation when a primary tumour is found in the thyroid
or as a retroperitoneal monodermal teratoma composed of
papillary cancer with anaplastic transformation [31]. We presented a very unique example of primary retroperitoneal cancer with ATC differentiation but there are known several other
similar cases. One published case represents a 75-year-old male
who had ATC developed between the sternocleidomastoid muscle and the common carotid artery. The thyroid had no changes
and it was totally separated from the tumour. The biopsy of the
thyroid or total thyroidectomy was not performed and histological analysis was not done. Possibly the ATC transformed
from papillary thyroid carcinoma to an extrathyroidal tumour.
Despite known poor survival, the patient lived 3.5 years without incidence of tumour recurrence after total resection and
around 5 years after the first symptoms [17]. Another studied
case of ATC manifestation in the extrathyroidal area was about
a 63-year-old female. She complained about a rapidly growing
abdominal mass during a month period and increased in size
neck mass over a week which caused dysphagia, shortness of
breath, and hoarseness. Important to note is that she had a history of goiter. The patient was diagnosed with ATC metastases
in the abdominal subcutaneous fat layer, and lungs. Palliative
radiotherapy was initiated for the neck area, and later systemic
chemotherapy was administrated. However, due to the aggressive nature of ATC, the patient experienced vocal cord paralysis
with severe airway problems and died around 9 weeks after the
hospitalization because of respiratory distress [32]. A similar situation was seen in a 64 year-old male who was examined due to
growing abdominal mass that was causing a mass effect on the
stomach, left adrenal gland, kidney, and pancreas. The patient
experienced total thyroidectomy 30 years ago because of papillary thyroid carcinoma and had an adjuvant radioactive iodine
treatment. Later metastases in the cervical lymph nodes and
left axilla were detected. The biopsy was taken from the growing retroperitoneal mass. According to the immunohistochemical profile, the results were similar to anaplastic transformation
in metastatic papillary thyroid cancer. The patient had a palliative resection surgery and died 3 weeks after the operation because of large bowel obstruction and sepsis [33].
ATC is associated with high aggressiveness because of fast
growth, high tendency for invasiveness, and low responsiveness
to most therapies [5,6]. ATC cases are staged as IV-stage thyroid
cancer due to ATC’s very aggressive nature as is followed by the
American Joint Committee on Cancer (AJCC) guidelines. Based
on the invasion, the extent of the tumour, and distant metastases, ATC is divided into stages: IVA stage (T1T3a, N0, M0) is
a tumour localized to the thyroid gland without lymph node
involvement (N0) and distant metastasis (M0). Stage IVB represents a primary tumour with gross extrathyroidal extension
(T3b,T4), and possible involvement of locoregional lymph nodes
(≥N1). IVC stage (any T, any N, M1) is a tumour with distant metastases (M1) [8].
Fine needle aspiration biopsy of a rapidly growing thyroid
or any other extrathyroidal mass or an abnormal lymph node
is the best method to diagnose ATC. However, it is difficult to
detect ATC in the early stage. Mostly ATC is diagnosed in an advanced stage, being a big mass, compressing surrounding structures like the trachea and causing symptoms such as dyspnoea,
dysphagia, neck pain, hoarseness, or other [2,8,22]. Nevertheless, ATC can also be detected incidentally during routine physical examination or urgent operations [2].
ATC is usually detected in older patients over 60 years
[2,8,18,23]. Also, it is more common in women than in men
[8,17,18,23]. Other known possible risk factors of this cancer
are obesity, a history of known thyroid nodular disease, and
malignancy in other sites such as prostate adenocarcinoma,
uterine cancer, and colic gastrointestinal stromal tumours [15].
There are more known factors that influence the survival of patients like distant metastases, socioeconomic status, and treatment strategy [2,8,15,19]. Local invasion is usually seen in ATC
cases. Also it is very common to detect metastasis in local or
distant lymph nodes, lungs, liver, and bones [15,16,22,24,25].
The aetiology of ATC is still unknown [2,9]. There are several hypotheses: it arises de novo or from the same mass of differentiated or poorly differentiated thyroid carcinoma [3,6,10].
Histological analysis of ATC is difficult since it is composed of
undifferentiated thyroid follicular cells and is likely to appear
in a multitude of microscopic variations [6,13,16]. Various immunohistochemical and ultrastructural analysis are made to determine their epithelial origin [15]. As for all malignant tumours
ATC has some certain features of high malignancy: invasiveness,
extensive tumour necrosis, marked nuclear pleomorphism, and
high mitotic activity [16,26]. The cell phenotype is known to be
of the epithelial-mesenchymal transition type [1]. Morphology of the cells might be different: pleomorphic giant, spindled
and squamous [16,27]. Also in some ATC cases paucicellular,
rhabdoid or small cell variants can be found [16,26]. All of this
makes ATC diagnosis more difficult and delayed [27]. Moreover,
an association has been found between ATC and accumulated
genetic alterations that are responsible for the regulation of the
MAPK and PI3K/AKT signalling pathways. These pathways affect
BARF, KRAS, PTEN and other genes that are known for several
modulatory cell functions: growth, survival and proliferation
[9,10].
The survival rate of ATC is very poor. According to the Research
Consortium of Japan, the median OS survival of the IVA, IVB and
IVC groups of patients was 15.8, 6.1, and 2.8 months respectively [11]. Surgical resection, radiation therapy, chemotherapy, or
a combination of different treatment options are used for ATC
patients [8]. The treatment strategy is known as one of the main
factors to predict prognosis [19]. Better survival is seen in cases after total resection of cancer with the combination of radiation
therapy chemotherapy/targeted therapy [12,23,28]. A multimodal approach is particularly recommended for IVA and IVB
stages. Palliative care and clinical trial are more often the treatment of choice for IVC stages [8]. The importance of the surgical
approach was shown in a retrospective review, 1-year survival
is much higher after total resection: 54% patients survived after
total resection of primary cancer, 28% – residual resection and
8 % without surgery [13]. A retrospective study from Finland
showed similar results. The median survival of patients with ATC
was 3.1 months. Longer survival was seen in the radical surgery
group than palliative, 11.6 and 3.2 months respectively. Also,
multimodal approach determined longer median survival (11.8
months) [29]. Historically doxorubicin was the most used agent
in chemotherapy for ATC treatment but new radiosensitizing
agents such as taxanes (paclitaxel or docetaxel), platin, cisplatin, and carboplatin seem to be more effective and can be used
alone or in combination [8]. In addition, these chemotherapy
agents are commonly used with radiation therapy [28]. Results
of molecular studies and the increased use of Tyrosine Kinase
Inhibitor (TKI) therapy are also giving promising results. According to the study of Park et al. 2021, the multimodal approach of
surgery, radiotherapy, and TKI therapy is the most effective and
resulting in a median survival of 34.3 months and in a 6-month
survival rate of 77.8%. Also, it is highlighted that more than half
of the patients showed a good response rate in the group treated only with TKI therapy [30]. Other treatment methods like
thyroid stimulating hormone suppression or radioiodine treatment are ineffective in ATC cases because this cancer does not
produce thyroglobulin and does not uptake iodine. Therefore
there are no tumour markers for ATC [3]. The treatment plan
of patients with ATC is recommended to be managed by a multidisciplinary team of endocrinologists, oncologists, surgeons,
radiotherapists, radiologists and psychologists [3,20]. Moreover
personalized multimodal course of treatment seems to be the
best approach for these patients [21].
Despite the improved prognosis of ATC over the past years
because of target therapy, immunotherapy and combination
of various treatment options, ATC remains a lethal disease
[19,20,28]. It is thought that airway problems and failures of
distant metastases control are one of the main causes of death
for ATC patients [30].
Conclusion
Anaplastic thyroid carcinoma is a very rare type of cancer.
Various factors such as metastatic stage, age, socioeconomic
status, treatment possibilities influence the prognosis. Multimodal approach and personalised treatment are recommended
for all anaplastic thyroid carcinoma. There are several clinical
cases of extrathyroidal tumours with anaplastic thyroid carcinoma differentiation. Our presented case represents a very rare
occurrence of anaplastic thyroid carcinoma in the retroperitoneal area. Even though almost 2 years have passed after the
initial diagnosis, the patient is still alive and represents a better
survival than is described in the literature. However, anaplastic thyroid carcinoma is a lethal disease and further studies are
needed for a better management and survival enhancement.
Declarations
Ethics approval and consent to participate: Ethics approval
was obtained and informed consent was gained from the patient.
Consent for publication: Consent for publication was gained
from the patient.
Availability of data and materials: The dataset supporting
the conclusions of this article is included within the article and
its additional files.
Competing interests: Not applicable.
Funding: Not applicable.
Authors’ contributions:
1. Ugne Imbrasaite - Investigation, Writing - original draft,
Visualization, Writing - Review & Editing, Resources.
2. Augustas Beisa - Conceptualization, Writing - Review &
Editing, Supervision.
3. Raminta Luksaite-Lukste - Review & Editing, Imaging analysis description.
4. Dmitrij Seinin - Review & Editing, Imaging analysis description
5. Laurynas Berzanskas - Writing - Review & Editing, Imaging
analysis - Description, Resources.
6. Pranas Serpytis - Review & Editing.
7. Tomas Poskus - Writing - Review & Editing, Supervision.
References
- B Lin ir kt, The incidence and survival analysis for anaplastic thyroid cancer: a SEER database analysis. 9.
- AV Chintakuntlawar RL. Foote JL, Kasperbauer ir KC.Bible Diagnosis and Management of Anaplastic Thyroid Cancer“, Endocrinol. Metab. Clin. North Am. 2019; 48(1): 269-284. kovo doi: 10.1016/j.ecl.2018.10.010.
- E Molinaro ir kt, Anaplastic thyroid carcinoma: from clinicopathology to genetics and advanced therapies, Nat. Rev. Endocrinol. 2017; 13(11): 644-660. lapkr. doi: 10.1038/nrendo.2017.76.
- G Nagaiah A, Hossain CJ, Mooney J, Parmentier, ir SC. Remick, Anaplastic Thyroid Cancer: A Review of Epidemiology, Pathogenesis, and Treatment J. Oncol. 2011; 1-13. doi: 10.1155/2011/542358.
- E. Kebebew FS, Greenspan OH, Clark KA, Woeber A. McMillan Anaplastic thyroid carcinoma: Treatment outcome and prognostic factors, Cancer t. 2005; 103(7): 1330-1335. doi: 10.1002/cncr.20936.
- SM Wiseman ir kt. Anaplastic transformation of thyroid cancer: Review of clinical, pathologic, and molecular evidence provides new insights into disease biology and future therapy, Head Neck, t. 2003 25(8): 662-670. 10.1002/hed.10277.
- T R, Liu ir kt. Treatment and Prognosis of Anaplastic Thyroid Carcinoma: A Clinical Study of 50 Cases PLOS ONE. 2016; 11(10). 0164840. 10.1371/journal.pone.0164840.
- KC Bible. American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer: American Thyroid Association Anaplastic Thyroid Cancer Guidelines Task Force“, Thyroid. 2021; 31(3): 337-386. doi: 10.1089/thy.2020.0944.
- H. Samimi. Molecular mechanisms of long non-coding RNAs in anaplastic thyroid cancer: a systematic review, Cancer Cell Int. 2020; 20(1): 352. doi: 10.1186/s12935-020-01439.
- T Kondo, S Ezzat, SL Asa. Pathogenetic mechanisms in thyroid follicularcell neoplasia, Nat. Rev. Cancer. 2006; 6(4): 292-306. 10.1038/nrc1836.
- N Onoda ir kt. Evaluation of the 8th Edition TNM Classification for Anaplastic Thyroid Carcinoma, Cancers. 2020; 12(3): 552. 10.3390/cancers12030552.
- S Ahmed. Imaging of Anaplastic Thyroid Carcinoma, Am J. Neuroradiol. 2018; 39(3): 547-551. doi: 10.3174/ajnr. A5487.
- A. Mohebati. Anaplastic Thyroid Carcinoma: A 25-year SingleInstitution Experience. 2014; 21(5): 1665-1670. doi: 10.1245/s10434-014-3545-5.
- RI. Haddad. Anaplastic Thyroid Carcinoma, Version 2. J Natl. Compr. Canc. Netw. 2015; 13(9): 1140-1150. doi: 10.6004/jnccn.2015.0139.
- G. Graceffa ir kt. Risk Factors for Anaplastic Thyroid Carcinoma: A Case Series From a Tertiary Referral Center for Thyroid Surgery and Literature Analysis, Front. 2022; 12: 948033. doi: 10.3389/fonc.2022.948033.
- X Du. Clinicopathological Characteristics of Mucinous Variant of Anaplastic Thyroid Carcinoma, Acta Endocrinol. Buchar. 2020; 16(3): 377-378. doi: 10.4183/aeb.2020.377.
- S Togashi ir kt. Thyroid anaplastic carcinoma transformed from papillary carcinoma in extrathyroid area, Auris. Nasus. Larynx, t. 2004; 31(3): 287-292. doi: 10.1016/j.anl.2004.03.006.
- Y. Zhou. A New Way Out of the Predicament of Anaplastic Thyroid Carcinoma from Existing Data Analysis, Front. Endocrinol. 2022; 13: 887906. doi: 10.3389/fendo.2022.887906.
- A Maniakas. Evaluation of Overall Survival in Patients with Anaplastic Thyroid Carcinoma. 2000-2019, JAMA Oncol. 2020; 6(9): 1397. doi: 10.1001/jamaoncol.2020.3362.
- S De Leo, M Trevisan, ir L Fugazzola, Recent advances in the management of anaplastic thyroid cancer, Thyroid Res. 2020; 13(1): 17. doi: 10.1186/s13044-020-00091-w.
- M Amaral, RA Afonso, MM Gaspar, CP Reis. Anaplastic thyroid cancer: How far can we go? EXCLI J. 19Doc800 ISSN. 2020; 1611-2156. doi: 10.17179/EXCLI2020-2257.
- T Kelil. Current Concepts in the Molecular Genetics and Management of Thyroid Cancer: An Update for Radiologists, RadioGraphics. 2016; 36(5): 1478-1493. doi: 10.1148/rg.2016150206.
- P Kasemsiri ir kt. Survival Benefit of Intervention Treatment in Advanced Anaplastic Thyroid Cancer, Int. J. Surg. Oncol. 2021; 1-8. doi: 10.1155/2021/5545127.
- J Simões-Pereira, R Capitão E. Limbert, ir V. Leite. Anaplastic Thyroid Cancer: Clinical Picture of the Last Two Decades at a Single Oncology Referral Centre and Novel Therapeutic Options, Cancers, t. 2019; 11(8): 1188. rugpj, doi: 10.3390/cancers11081188.
- T Abe. Anaplastic transformation of papillary thyroid carcinoma in multiple lung metastases presenting with a malignant pleural effusion: a case report, J. Med. Case Reports. 2014; 8(1): 460. doi: 10.1186/17521947-8-460.
- A Deeken-Draisey, GY. Yang, J Gao, BA Alexiev. Anaplastic thyroid carcinoma: an epidemiologic, histologic, immunohistochemical, and molecular single-institution study, Hum. Pathol. 2018; 82: 140-148. gruodž. doi: 10.1016/j.humpath.2018.07.027.
- ME Cabanillas, DG. McFadden, ir C. Durante.Thyroid cancer, The Lancet. 388, nr. 2016; 10061: 2783-2795. doi: 10.1016/S0140-6736(16)30172-6.
- N Huang. An Update of the Appropriate Treatment Strategies in Anaplastic Thyroid Cancer: A Population-Based Study of 735 Patients, Int. J. Endocrinol. 2019; 1-7 doi: 10.1155/2019/8428547.
- P Siironen. Anaplastic and Poorly Differentiated Thyroid Carcinoma: Therapeutic Strategies and Treatment Outcome of 52 Consecutive Patients, Oncology t. 2010; 79(5-6): 400-408. doi: 10.1159/000322640.
- J Park. Multimodal treatments and outcomes for anaplastic thyroid cancer before and after tyrosine kinase inhibitor therapy: a real-world experience, Eur J. Endocrinol. 2010; 184(6): 837-845. doi: 10.1530/EJE-20-1482.
- A Nourparvar, J Lechago, GD. Braunstein. Thyroid Carcinoma Arising from a Sacrococcygeal Mass: A Malignant Teratoma? Thyroid. 2004; 14(7): 548-552. liep., doi: 10.1089/1050725041517101.
- KH Lim, KW Lee, JH Kim, SY Park, SH Choi, JS Lee. Thyroid Carcinoma Initially Presented with Abdominal Cutaneous Mass and Hyperthyroidism, Korean J. Intern. 2010; 25(4): 450. doi: 10.3904/kjim.2010.25.4.450.
- JP Solomon, F Wen, LJ Jih. Anaplastic Transformation of Papillary Thyroid Cancer in the Retroperitoneum, Case Rep. 2015. 1-4. doi: 10.1155/2015/241308.